1. Yu-Nong Li*, Xiao-Fang Liu and Liang-Nian He*, An alternative route of CO2 conversion: Pd/C-catalyzed oxazolidinone hydrogenation to HCOOH and secondary alkyl-(2-arylethyl)amines with one stone two bird strategy. J. CO2 Util., 2019, 29, 74-81. [link]

Abstract: The oxazolidinone as one kind of annular CO2 derivatives has been hydrogenated by a new catalytic system Pd/C-TMGPEG150Me, affording formic acid and the corresponding alkyl-(2-arylethyl)amines under mild conditions in the high yields of 89% and 98%, respectively. This one stone two bird strategy validates a potential alternative route of approach for indirect conversion of CO2 to energy-related products, and opens up a novel way of synthesizing linear secondary amines without selectivity issues.
2. Shuai Zhang*, Feng Han, Shaorui Yan, Mingyue He, Chengxia Miao and Liang-Nian He*, Efficient Catalysts In Situ Generated from Zinc, Amide and Benzyl Bromide for Epoxide/CO2 Coupling Reaction at Atmospheric Pressure. Eur. J. Org. Chem., 2019, 2019, 1311-1316. [link]

Abstract: Active catalysts in situ generated from Zn powder, dimethyl formamide and benzyl bromide were designed for fixation of CO2 to cyclic carbonates at 80 oC and atmospheric pressure of CO2. Zinc bromide and N,N-dibenzyl-N,N-dimethylammonium bromide, being proved as active catalyst species, were in situ generated and immediately converted various terminal epoxides to the corresponding carbonates efficiently.
3. Xian-Dong Lang, Zheng-Ming Li*, and Liang-Nian He*, Protic ionic liquid-catalyzed synthesis of oxazolidinones using cyclic carbonates as both CO2 surrogate and sustainable solvent. Catal. Today, 2019, 324, 167-173. [link]

Abstract: The thermodynamic stability and kinetic inertness represent substantial obstacles for direct chemical transformation of CO2. Consequently, conversion of the CO2 equivalent in an efficient and energy-saving manner has gained much attention being viewed as an indirect pathway to chemical utilization of CO2. In this article, we would like to report the synthesis of oxazolidinones via the carboxylative cyclization of anilines with cyclic carbonate using protic ionic liquid 1,8-diazabicyclo[5.4.0]-7-undecenium imidazolide [HDBU][Im] as the bifunctional catalyst under mild conditions without addition of conventional organic solvents. The distinguished features of this protocol include low catalyst loading, wide functionality tolerance and convenient recycling of the catalyst. A series of functional groups such as Cl, Br, CH3, OCH3 and NO2 can be well tolerated under the reaction conditions, providing the corresponding products in moderate to good yields (61–92%). In addition, to shed light on the cooperative interactions of [HDBU][Im]/EC and [HDBU][Im]/aniline, NMR technique and DFT study were also performed, respectively.
4. Xian-Dong Lang, Fei You, Xing He, Yi-Chen Yu and Liang-Nian He*, Rhodium(I)-catalyzed Pauson–Khand-type reaction using formic acid as a CO surrogate: an alternative approach for indirect CO2 utilization. Green Chem., 2019, 21(3), 509-514. [link]

Abstract: Formic acid is found to be an ideal CO surrogate for the rhodium(I)-catalyzed Pauson–Khand-type (PK-type) reaction of various substituted 1,6-enynes to afford bicyclic cyclopentenones in moderate to good yields. High TON value of up to 263 and good results in the gram-scale experiment were also obtained, demonstrating the efficacy of this methodology. In addition, heterocyclic molecules of pharmaceutical importance were also furnished via inter- or intra-molecular hetero-PK-type reactions, further broadening the application of current strategy. In this protocol, formic acid was utilized as a bridging molecule for the conversion of CO2 to CO, since formic acid is manufactured via catalytic hydrogenation of CO2 and releases CO in the presence of acetic anhydride readily. Therefore, this methodology represents a green and indirect approach for chemical valorization of CO2 in the preparation of value-added compounds.
5. Xiaoya Li and Liang-Nian He*, Ionic Liquid-Promoted CO2 Reductive Functionalization. In Encyclopedia of Ionic Liquids, Zhang, S., Ed. Springer Singapore: Singapore, 2019; pp 1-7. [link]
6. Shumei Xia and Liang-Nian He*, Ionic Liquids in Nucleophilic Substitution. In Encyclopedia of Ionic Liquids, Zhang, S., Ed. Springer Singapore: Singapore, 2019; pp 1-8. [link]
7. Xiao-Fang Liu, Xiao-Ya Li and Liang-Nian He*, Transition Metal-Catalyzed Reductive Functionalization of CO2. Eur. J. Org. Chem., 2019, 2019(14), 2437-2447. (VIP Paper) [link]

Abstract: Reductive functionalization of CO2 combining both the formation of the new bonds and CO2 reduction in the presence of reductant, such as molecular hydrogen, hydrosilane, or hydroborane has become increasingly attractive, which enlarges the spectra of compounds directly available from CO2 thus provides fresh idea for CO2 chemistry. This microreview briefly summarizes recent advances in new bond construction using CO2 as formyl, methylene, and methyl source with transition‐metal catalyst, which are divided into sections according to C–N, C–C, and C–O bonds formation in the presence of nitrogen‐, carbon‐, and oxygen‐nucleophiles respectively. The challenges and opportunities with future trends of the reductive functionalization of CO2 are discussed as well.
8. Zhi-Hua Zhou, Xiao Zhang, Yong-Fu Huang, Kai-Hong Chen* and Liang-Nian He*, Synthesis of α-hydroxy ketones by copper(I)-catalyzed hydration of propargylic alcohols: CO2 as a cocatalyst under atmospheric pressure. Chin. J. Catal., 2019, 40(9), 1345-1351. [link]

Abstract: Inexpensive and efficient Cu(I) catalysis is reported for the synthesis of α-hydroxy ketones from propargylic alcohols, CO2, and water via tandem carboxylative cyclization and nucleophilic addition reaction. Notably, hydration of propargylic alcohols can be carried out smoothly under atmospheric CO2 pressure, generating a series of α-hydroxy ketones efficiently and selectively. This strategy shows great potential for the preparation of valuable α-hydroxy ketones by using CO2 as a crucial cocatalyst under mild conditions.
9. Liang-Nian He (Editor-in-Chief), Preface for Current Organic Synthesis, 2019, 16(1), 2. [link]
10. Liang-Nian He*, Response to Commentary by T. Mita on Transition Metal-Catalyzed Carboxylation of Terminal Alkynes with CO2. Mini-Reviews in Organic Chemistry, 2019, 16(5), 409. [link]
11. Hong-Chen Fu, Fei You, Hong-Ru Li* and Liang-Nian He*, CO2 Capture and in situ Catalytic Transformation. Front. Chem., 2019, 7(525), doi: 10.3389/fchem.2019.00525. [link]
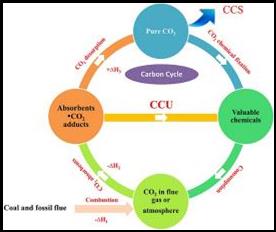
Abstract: The escalating rate of fossil fuel combustion contributes to excessive CO2 emission and the resulting global climate change has drawn considerable attention. Therefore, tremendous efforts have been devoted to mitigate the CO2 accumulation in the atmosphere. Carbon capture and storage (CCS) strategy has been regarded as one of the promising options for controlling CO2 build-up. However, desorption and compression of CO2 need extra energy input. To circumvent this energy issue, carbon capture and utilization (CCU) strategy has been proposed whereby CO2 can be captured and in situ activated simultaneously to participate in the subsequent conversion under mild conditions, offering valuable compounds. As an alternative to CCS, the CCU has attracted much concern. Although various absorbents have been developed for the CCU strategy, the direct, in situ chemical conversion of the captured CO2 into valuable chemicals remains in its infancies compared with the gaseous CO2 conversion. This review summarizes the recent progress on CO2 capture and in situ catalytic transformation. The contents are introduced according to the absorbent types, in which different reaction type is involved and the transformation mechanism of the captured CO2 and the role of the absorbent in the conversion are especially elucidated. We hope this review can shed light on the transformation of the captured CO2 and arouse broad concern on the CCU strategy.
12. Zhi-Hua Zhou, Shu-Mei Xia, Si-Yuan Huang, Yu-Zhong Huang, Kai-Hong Chen* and Liang-Nian He*, Cobalt-based catalysis for carboxylative cyclization of propargylic amines with CO2 at atmospheric pressure. J. CO2 Util., 2019, 34, 404-410. [link]

Abstract: A cobalt-bicyclic guanidine catalytic system consisting of CoBr2 and 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) for the carboxylative cyclization of terminal propargylic amines with CO2 was firstly developed in this work to produce 2-oxazolinones efficiently. The existence of induction period urged us to understand the reaction mechanism of cobalt catalysis. Investigation on the roles of CoBr2 and TBD was conducted using control experiments and density functional theory (DFT) calculation. TBD presumably acts as a base to activate propargylic amine for favorable CO2 capture, and a ligand to coordinate with CoBr2 via forming bulkier CoBr2(TBD) in the same time. This bulkier complex can enhance the O-nucleophility of the in situ formed carbamate intermediate and then promote subsequent intramolecular cyclization to generate 2-oxazolinone, accounting for the high activity of cobalt catalysis. This protocol enables the synthesis of various 2-oxazolinones from propargylic amines and CO2 under atmospheric pressure in good to excellent yields, representing a simple, cost-effective and practical route for CO2 fixation to 2-oxazolinones under mild conditions.
13. Zhi-Hua Zhou, Kai-Hong Chen,* and Liang-Nian He*, Efficient and Recyclable Cobalt(II)/Ionic Liquid Catalytic System for CO2 Conversion to Prepare 2-Oxazolinones at Atmospheric Pressure, Chin. J. Chem. 2019, 37, 1223-1228.

Abstract: Converting CO2 into value-added chemicals represents a promising way to alleviate the CO2 derived environmental issues, for which the development of catalysts with high efficiency and recyclability is very desirable. Herein, the catalytic system by combining cobalt source and ionic liquid (IL) has been developed as the efficacious and recyclable catalyst for the carboxylative cyclization of propargylic amine and CO2 to prepare 2-oxazolinones. In this protocol, various propargylic amines were successfully transformed into the corresponding 2-oxazolinones with CoBr2 and diethylimidazolium acetate ([EEIM][OAc]) as the catalyst under atmospheric CO2 pressure. It is worth noting that the turnover number (TON) of this transformation can be up to 1740, presumably being attributed to the cooperative effect of the cobalt and IL. Furthermore, the existence of IL enables the catalytic system to be easily recycled to 10 times without losing its activity.
14. Xing He, Xiang‐Yang Yao, Kai‐Hong Chen* and Liang‐Nian He*, Metal‐Free Photocatalytic Synthesis of exo‐Iodomethylene 2‐Oxazolidinones: An Alternative Strategy for CO2 Valorization with Solar Energy, ChemSusShem, 2019, 12, 5081-5085. [link]

Abstract: A visible-light-promoted metal-free carboxylative cyclization of propargylic amines with CO2 was shown to offer exo-iodomethylene 2-oxazolidinones. Incorporation of both CO2 and iodo moieties into these compounds was realized efficiently. The mechanism study revealed that this carboxylative cyclization proceeds through a radical pathway. Notably, the iodine-functionalized 2-oxazolidinone as a platform molecule could be easily converted into a wide range of value-added chemicals through Buchwald-Hartwig, Suzuki, Sonogashira, photocatalytic ene, and photoreduction reactions. As a result, the plentiful downstream transformations remarkably enhance the range of chemicals derived from CO2 and open a potential avenue for CO2 functionalization to circumvent energy challenges in this field.
15. 姚向阳, 张彦, 高嵩, 何良年*. 面向可持续发展的二氧化碳化学研究进展. 华中师范大学学报(自然科学版), 2019, 53(6), 834-846, DOI: 10.19603/j.cnki.1000-1190.2019.06.002.

摘 要:二氧化碳既是温室气体的主要成分又是储量丰富且可再生的碳资源,研究二氧化碳的化学转化和利用对可持续发展具有重要意义。二氧化碳的热力学稳定性和动力学惰性决定了发展高效催化体系是活化二氧化碳的关键。围绕二氧化碳介质中的化学反应、二氧化碳原位酸催化反应、二氧化碳的捕集与利用、可见光催化的二氧化碳的利用、二氧化碳作为C1合成子构筑C-C/C-N/C-O键以及二氧化碳的还原功能化等方面系统地介绍了本课题组在二氧化碳化学方面的研究,并分析面临的挑战、对策以及发展趋势。