1. Xiao-Fang Liu, Xiao-Ya Li, Chang Qiao, Hong-Chen Fu, Liang-Nian He*, Betaine Catalysis for Hierarchical Reduction of CO2 with Amine and Hydrosilane to Formamide, Aminal and Methylamine, Angew. Chem. Int. Ed., 2017, 56, 7425-7429. [link]

Abstract: An efficient, sustainable organocatalyst e.g. glycine betaine was developed for reductive functionalization of CO2 with amines and diphenylsilane. Methylamines and formamides could respectively be obtained with high yield via tuning CO2 pressure and reaction temperature. Betaine catalysis was efficient for the formation of formamide at 10 bar CO2, 50 oC. Exclusively yielding methylamines from various amines with atmospheric pressure of CO2 was attained at 70 oC. Based on identification of the key intermediate i.e. aminal, an alternative mechanism for methylation involving the C0 silyl acetal and aminal was proposed. Furthermore, reducing CO2 amount to approx. 1 equiv. afforded aminal with high yield and selectivity. Therefore, betaine catalysis in this study afforded the products with diversified energy content i.e. formamide, aminal and methylamine respectively through hierarchical 2-, 4- and 6-electron reduction of CO2 coupled with C-N bond formation for the first time.
2. Xi Liu, Mei-Yan Wang, Si-Yuan Wang, Qi Wang, Liang-Nian He*, Zinc(II) Catalyst In Situ Formed for Incorporation of CO2 into 2-Oxazolidinones with Propargylic Amines at Atmospheric Pressure, ChemSusChem, 2017, 10, 1210-1216. [link]

Abstract: Incorporation of CO2 into heterocyclic compounds (i.e., 2-oxazolidinones) under mild conditions, especially at atmospheric pressure still remains challenging. The mononuclear ZnII complex ZnCl2(TBD)2, where TBD=1,5,7-triazabicyclo[4.4.0]dec-5-ene, in this study was demonstrated as a robust catalyst for the carboxylative cyclization of propargylic amines with CO2 to exclusively afford various 2-oxazolidinones in excellent yields. Notably, the ZnII catalytic species is readily generated in situ from ZnCl2 and TBD without pre-preparation and further isolation. Such a CO2 fixation protocol could proceed smoothly under atmospheric pressure at mild temperature in an atom economic and environmentally benign manner. 13C NMR and control experiments were performed to explore the possible interaction between ZnII and the carbon–carbon triple bond of propargylic amine. The dual catalytic role of the Zn catalyst to enhance O-nucleophilicity of the carbamate anion intermediate and activate the carbon–carbon triple bond is proposed based on mechanistic investigations.
3. Mei-Yan Wang, Yu Cao, Xi Liu, Ning Wang, Liang-Nian He* and Si-Han Li, Photoinduced radical-initiated carboxylative cyclization of allyl amines with carbon dioxide. Green Chem., 2017, 19, 1240-1244. [link]

Abstract: Visible light-promoted CO2 upgrading: a highly efficient and metal-free photochemical method for the carboxylative cyclization of allyl amines with CO2 is reported to prepare perfluoroalkylated oxazolidinones with high efficiency under ambient conditions by using perfluoroalkyl iodides as radical sources.
4. Zhi-Hua Zhou, Qing-Wen Song, and Liang-Nian He*, Silver(I)-Promoted Cascade Reaction of Propargylic Alcohols, Carbon Dioxide, and Vicinal Diols: Thermodynamically Favorable Route to Cyclic Carbonates, ACS Omega, 2017, 2, 337-345. [link]

Abstract: A silver(I)-promoted cascade reaction was developed for the synthesis of cyclic carbonates from terminal propargylic alcohols, carbon dioxide, and vicinal diols. Compared with direct condensation of vicinal diols with CO2, this protocol provides a thermodynamically favorable route to cyclic carbonates and α-hydroxyl ketones in excellent yields (up to 97%) without the additional dehydration step. Such a cascade procedure proceeds presumably through initial reaction of propargylic alcohol with CO2 and subsequent nucleophilic attack of vicinal alcohol on in situ-formed α-alkylidene cyclic carbonate, resulting in successive generation of α-alkylidene cyclic carbonate, unsymmetrical β-oxoalkyl carbonate, cyclic carbonate, and α-hydroxyl ketone.
5. Xi Liu, Liang-Nian He*, Synthesis of Lactones and Other Heterocycles in Topical Collection “Chemical Transformations of Carbon Dioxide”; Series Editors: Xiao-Feng Wu, Matthias Beller. Topics in Current Chemistry (ISSN: 2365-0869 (Print) 2364-8961 (Online)), Springer International Publishing. (2017) 375: 21. [link]
Abstract: Chemical fixation of CO2 into value-added chemicals represents a promising field in view of sustainable development and green synthesis. In this aspect, the construction of heterocyclic compounds from CO2 and readily available starting materials is particularly appealing in both organic and pharmaceutical fields since CO2 can be regarded as carbon and oxygen resource with advantages of abundance, renewability, non-toxicity, and non-flammability. In this chapter, we have summarized elegant protocols with elaborately designed substrates for the direct incorporation of entire CO2 molecule or “CO” or “C” fragments into lactones and other heterocycles such as oxazolidinones, cyclic carbonates, quinazoline-2,4(1H,3H)-diones, etc., through the formation of carbon–carbon, carbon–nitrogen and/or carbon–oxygen bonds promoted by homogeneous catalysts.
6. Xuedong Li, Xing He, Xiaofang Liu, Liang-Nian He*, Ruthenium-promoted reductive transformation of CO2, Sci China Chem, 2017, 60, 841-852. [link]

Abstract: The reductive transformation of CO2 to energy related products including formic acid, CO, formamide, methanol and methylamine could be a promising option to supply renewable energy. In this aspect, ruthenium has found wide application in hydrogenation of various carbonyl groups, and has successfully been applied to reductive transformation of CO2 with high catalytic efficiency and excellent selectivity. In addition, ruthenium complexes have also served as effective photosensitizers for CO2 photoreduction. Classified by reductive products, this review summarizes and updates advances on the Ru-catalyzed reduction of CO2 along with catalyst development on the basis of mechanistic understanding at a molecular level.
7. Xiao-Fang Liu, Mei-Yan Wang and Liang-Nian He*, Heterogeneous Catalysis for Oxazolidinone Synthesis from Aziridines and CO2, Current Organic Chemistry, 2017, 21, 698-707. [link]

Abstract: With the increasing environmental and societal concerns about global warming associated with carbon emission, great efforts have been devoted to carbon dioxide fixation during the past two decades. Indeed, CO2 has been widely used as a potential C1 building block for the production of various chemical products, as it is nontoxic, renewable, economical and abundant. One promising methodology is the coupling reaction of CO2 with aziridines to afford oxazolidinones, which is 100% atom-efficient and eco-friendly. In addition, oxazolidinones are important heterocyclic compounds showing application as intermediates and chiral auxiliaries in organic synthesis. To date, numerous homogeneous catalysts have been developed for this reaction. With criteria of green chemistry and sustainable development, developing efficient heterogeneous catalysts would be crucial for industrial application. In this review article, we present the latest progress on the coupling reaction of CO2 with aziridines, and particularly focus on the preparation, activity evaluation and recyclability of various heterogeneous catalytic systems including mesoporous material, functionalized polymer-based catalysts and supported catalysts. We hope this review could stimulate further research on oxazolidinone synthesis from CO2 and aziridines with green and efficient heterogeneous catalyst.
8. Editorial Overview: Liangnian He, Thibault Cantat, Innovative methods in CO2 conversion: A breath of fresh air? Current Opinion in Green and Sustainable Chemistry, 2017, DOI: 10.1016/j.cogsc.2017.01.001. [link]
9. Liang-Nian He (Editor-in-Chief), Preface for Current Organic Synthesis, 2017, 14(1), 2. [link]
10. Chang Qiao, Xiao-Fang Liu, Xi Liu, and Liang-Nian He*, Copper(II)-Catalyzed Selective Reductive Methylation of Amines with Formic Acid: An Option for Indirect Utilization of CO2, Org. Lett., 2017, 19, 1490-1493. [link]

Abstract: A copper-catalyzed protocol for reductive methylation of amines and imine with formic acid as a C1 source and phenylsilane as a reductant is reported for the first time, affording the corresponding methylamines in good to excellent yields under mild conditions. This protocol offers an alternative method for indirect utilization of CO2, as formic acid can be readily obtained from hydrogenation of CO2.
11. Ran Ma, Liang-Nian He*, Xiao-Fang Liu, Xi Liu and Mei-Yan Wang, DBU as activator for the N-iodosuccinimide promoted chemical fixation of carbon dioxide with epoxides. J. CO2 Util., 2017, 19, 28-32. [link]

Abstract: The combination of N-Iodosuccinimide (NIS) and DBU is developed as an efficient organocatalyst system for the cycloaddition of carbon dioxide (CO2) at atmospheric pressure with epoxides without utilization of additional solvents, forming cyclic carbonates in high yields with a broad substrate scope. DBU functions as a nucleophilic promoter for the activation of NIS to be a more electrophilic iodine species thus being capable of activating the epoxides. On the other hand, NIS also provides a nucleophilic nitrogen species, i.e. succinimide anion for ring-opening of the epoxide.
12. Xiao-Fang Liu, Chang Qiao, Xiao-Ya Li and Liang-Nian He*, Carboxylate-promoted reductive functionalization of CO2 with amines and hydrosilanes under mild conditions, Green Chem., 2017, 19, 1726-1731. [link]

Abstract: Various oxygen-nucleophiles especially carboxylates, e.g. cesium/tetrabutylammonium carboxylate, were proved to be efficient and selective catalysts for reductive functionalization of CO2 with amines and hydrosilanes to methylamines. Various amines including aromatic and aliphatic, primary and secondary ones were methylated successfully in the presence of diphenylsilane as the reductant under 50 °C and an atmospheric pressure of CO2. Furthermore, a reaction pathway involving CO2 reduction to the C0 species i.e. aminal rather than the formamide as the intermediate was proposed. This protocol represents a transition metal-free and environmentally friendly option for CO2 conversion to useful chemicals via the formation of C–N bonds coupled with six-electron reduction of CO2 to the methanol level under mild conditions.
13. Qing-Wen Song, Zhi-Hua Zhou and Liang-Nian He*, Efficient, selective and sustainable catalysis of carbon dioxide, Green Chem., 2017, 19, 3707-3728. [link]

Abstract: Performing CO2 conversion in a cost-effective and environmentally benign manner would be promising and remains still challenging due to its thermodynamic stability and kinetic inertness. Herein, we would like to update significant advances in organic synthesis using CO2 with high catalytic efficiency and excellent selectivity towards the target product mainly during latest five years (2012-2016). Achieving an efficient and selective CO2 conversion depends on development of metal catalysts (especially functional metal complex catalysis) including main-group metal, typical transition metal and lanthanide series metal as well as organocatalysts e.g. N-heterocyclic carbene, N-heterocyclic olefin, task-specific ionic liquid, superbase and frustrated Lewis pair being able to effectively activate CO2 and/or the substrate on the basis of mechanistic understanding at a molecular level. This review just covers typical catalytic transformation of CO2, for instance, carboxylation, amidation, hydrogenation, and representative green processes like solvent-less, halogen-free that use CO2 as an ideal carbon-neutral source to prepare valuable compounds with the improved atom economy and enhanced sustainability of chemical processes through green catalysis. In particular, in situ catalytic CO2 conversion, i.e. the combination of carbon capture and subsequent conversion, a recent breakthrough in CO2 chemistry field is also discussed.
14. Gang Xiong, Bing Yu, Jie Dong, Ying Shi, Bin Zhao* and Liang-Nian He*, Cluster-based MOFs with Accelerating Chemical Conversion of CO2 through C-C Bond Formation, Chem. Commun., 2017, 53, 6013-6016. [link]

Abstract: Investigations on metal-organic frameworks (MOFs) as direct catalysts have been well documented, but it has never been reported that MOFs directly catalyze the chemical conversion of terminal alkynes and CO2 as chemical feedstock into valuable chemical products. We report here two cluster-based MOFs I and II assembled by multinuclear Gd-cluster and Cu-cluster, displaying high thermal and solvent stabilities. I and II as heterogeneous catalysts possess active catalytic centers [Cu12I12] and [Cu3I2], respectively, exhibiting excellent catalytic performance in the carboxylation reactions of CO2 and 14 kinds of terminal alkynes under 1 atm and mild conditions. This is the first time for MOFs materials to catalyze the carboxylation reaction of terminal alkynes and CO2 without any cocatalyst/additive. This work not only reduces greenhouse gas emission but also provides high valuable materials, opening a wide space in seeking recoverable catalysts to accelerate chemical conversion of CO2.
15. Xue-Dong Li, Qing-Wen Song, Xian-Dong Lang, Liang-Nian He*, Yao Chang, Ag(I)/TMG-Promoted Cascade Reaction of Propargyl Alcohols, Carbon Dioxide, and 2-Aminoethanols to 2-Oxazolidinones, ChemPhysChem, 2017, 18, 3182-3188. [link]
Abstract: Chemical valorization of CO2 to access various value-added compounds has been a long-term and challenging object from the viewpoint of sustainable chemistry. Herein, a one-pot three-component reaction of terminal propargyl alcohols, CO2, and 2-aminoethanols was developed for the synthesis of 2-oxazolidinones and equal amount of α-hydroxyl ketones promoted by Ag2O/TMG (1,1,3,3-tetramethylguanidine) with the TON (turnover number) up to 1260. By addition of terminal propargyl alcohol, thermodynamic disadvantage of the conventional 2-aminoethanol/CO2 coupling was ameliorated. Mechanistic investigations including control experiments, kinetic and NMR studies suggest that the reaction proceeds through a cascade pathway and TMG could activate propargyl alcohol and 2-aminoethanol through the formation of hydrogen bonding and also activate CO2.
16. Xian-Dong Lang, Xing He, Zheng-Ming Li* and Liang-Nian He*, New routes for CO2 activation and subsequent conversion, Current Opinion in Green and Sustainable Chemistry, 2017, 7, 31-38. [link]

Abstract: Nowadays, transformation of CO2 into value-added chemicals and fuels has attracted much attention. However, the inherent thermodynamic and kinetic limitation of CO2 represents the biggest obstacle associated with CO2 conversion. In this context, activation of CO2 would be prerequisite for its conversion. In this minireview, we would like to provide a concise overview of recent advances on CO2 activation by various organocatalysts including N-heterocyclic carbenes (NHCs)/N-heterocyclicolefins (NHOs), phosphorus ylides, polyoxometalates (POMs), ionic liquids (ILs), frustrated Lewis pairs (FLPs), superbases. In addition, carbon capture and utilization (CCU) strategy, the elegant combination of carbon capture and subsequent conversion, has also been summarized, which is designed to obviate the high energy input associated with carbon capture and storage/sequestration (CCS) process. In order to demonstrate the efficiency of CO2 activation, application of abovementioned CO2 activation in the synthesis of cyclic carbonates, carbon monoxide, quinazoline-2,4(1H,3H)-diones, methanol, oxazolidinones, formamides, ureas, alkyl 2-alkynoates, α-hydroxy ketones, and formic acid are also included.
17. Ke Shi, Liefeng Feng, Liang-Nian He, Hongru Li*, Solubility Determination and Correlation of Gatifloxacin, Enrofloxacin, and Ciprofloxacin in Supercritical CO2, J. Chem. & Eng. Data, 2017, 62, 4235-4243.
18. Ke Shi, Liefeng Feng, Liang-Nian He, Hongru Li*, Thermodynamic modeling of the supercritical CO2 impregnation process for the preparation ibuprofen/polymethylmethacrylate composite, J. Taiwan Inst. Chem. Eng., 2017, 78, 471-476.
19. Zhi-Hua Zhou, Chun-Xiang Guo, Jia-Ning Xie, Kai-Xuan Liu, and Liang-Nian He*, Silver Chloride/Triphenylphosphine-Promoted Carboxylation of Arylboronic Esters with Carbon Dioxide at Atmospheric Pressure, Current Org. Synth., 2017, 14, 1185-1192. [link]

Abstract: The carboxylation of arylboronic esters with ambient CO2 was achieved based on silver chloride/triphenylphosphine system. To achieve this transformation under atmospheric CO2 pressure, the effect of base and solvent was investigated and cesium carbonate and dimethyl sulfoxide were confirmed as the most appropriate base and solvent. Through this protocol, various functionalized carboxylic acids were synthesized in 85-99% yields. In addition, C-13 isotope labeling experiment confirmed the exclusive carboxyl source from CO2 rather than cesium carbonate.
20. Xing He, Xiao-Ya Li, Yu Song, Shu-Mei Xia, Xian-Dong Lang and Liang-Nian He*, Synthesis of Urea Derivatives Using Carbon Dioxide as Carbonylation Reagent in Ionic Liquids, Current Organocatal., 2017, 4 (2), 112-121. [link]
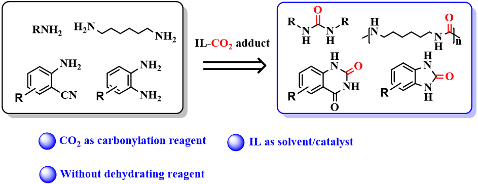
Abstract: Urea and its derivatives, which are usually generated through the carbonylation reaction between amines and carbonylation reagent, have been found widespread applications in agriculture and pharmaceuticals. Among the carbonylation reaction, it is the most appealing and promising strategy that employing CO2 as a green carbonylation reagent. However, CO2 inherent thermodynamic stability and kinetic inertness limit its application. Apart from being regarded as one of green solvents, ionic liquid is also an efficient organocatalyst for CO2 capture or activation due to the interaction between CO2 with cation or anion of ionic liquid. In this minireview, we have summarized representative synthetic methodologies of urea derivatives using carbon dioxide as a green carbonylation reagent and using ionic liquids as solvents and/or organocatalysts.