1. Qing-Wen Song and Liang-Nian He*, Robust Silver(I) Catalyst for the Carboxylative Cyclization of Propargylic Alcohols with Carbon Dioxide under Ambient Conditions, Adv. Synth. Catal., 2016, 358(8), 1251-1258. [Selected as VIP Paper]. [link]

Abstract: Inspired by the bulkier bis(triphenylphosphine)–silver cation-induced mechanism of propargylic alcohols and carbon dioxide through the alkyl carbonate intermediate, a robust dual-component catalytic system consisting of silver acetate and tetraheptylammonium bromide was rationally developed for the synthesis of α-methylene cyclic carbonates under ambient conditions without employing any additional organic base and ligand. This is one of the most effective catalysts reported to date for this conversion, with a very high turnover number of up to 6024, probably due to the synergistic effect of Lewis basic and Lewis acidic species for the activation of both propargylic alcohol and carbon dioxide by the formation of the alkyl carbonate with a bulkier counterion. Notably, this catalyst also worked well for the carboxylative cyclization of propargylic amines with carbon dioxide with the highest turnover number of 544.

2. Ran Ma, Liang-Nian He* and Yue-Biao Zhou, An efficient and recyclable tetraoxo-coordinated zinc catalyst for the cycloaddition of epoxides with carbon dioxide at atmospheric pressure, Green Chem., 2016, 18(1), 226-231. [link]
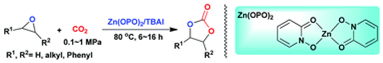
Abstract: An efficient and recyclable catalytic system of a Zn(O)4-coordinated complex e.g. 2-hydroxypyridine N-oxide zinc(II), Zn(OPO)2 and tetrabutylammonium iodide (TBAI) was developed for the cyclic carbonate synthesis from epoxides and CO2 without the use of any organic solvents. This easily prepared, low-toxic Zn(OPO)2 may have a strong Lewis acidity for the activation of epoxides and exhibited high activity (TOF up to 22000 h−1) even at a CO2 pressure as low as 1 bar with a broad substrate scope. Furthermore, the catalyst can be easily recovered and reused five times without a significant loss of its catalytic activity.
3. Mei-Yan Wang, Qing-Wen Song, Ran Ma, Jia-Ning Xie and Liang-Nian He*, Efficient conversion of carbon dioxide at atmospheric pressure to 2-oxazolidinones promoted by bifunctional Cu(II)-substituted polyoxometalate-based ionic liquids, Green Chem., 2016, 18, 282-287. [link]

Abstract: Copper(II) substituted polyoxometalate-based ionic liquids e.g. [(nC7H15)4N]6[α-SiW11O39Cu] were successfully developed as halogen-free bifunctional catalysts for the carboxylative cyclization of propargylic amines with CO2. Such a CO2 fixation protocol proceeded smoothly at atmospheric pressure under solvent-free conditions, in an environmentally benign and low energy-input manner. Notably, various propargylic amines could react smoothly to afford 2-oxazolidinones as the target products in high to quantitative yields. Furthermore, the dual activation of both propargylic amine and CO2 by [(nC7H15)4N]6[α-SiW11O39Cu] was studied using NMR techniques and control experiments.
4. Ran Ma, Liang-Nian He,* An-Hua Liu and Qing-Wen Song, Cu(II)-catalyzed esterification reaction via aerobic oxidative cleavage of C(CO)–C(alkyl) bonds, Chem. Commun., 2016, 52, 2145-2148. [link]
Abstract: A novel Cu(II)-catalyzed aerobic oxidative esterification of simple ketones for the synthesis of esters has been developed with wide functional group tolerance. This process is assumed to go through a tandem sequence consisting of α-oxygenation/esterification/nucleophilic addition/C–C bond cleavage and carbon dioxide is released as the only byproduct.
5. Shuai Zhang, Ran Ma, Liang-Nian He*, Transition Metal-Free Incorporation of CO2 in Carbon Dioxide and Organometallics, Vol. 53, Springer International Publishing, 2016, pp. 143-169. [link]
Abstract: Carbon dioxide can be regarded as an ideal C1 chemical feedstock in both academic and pharmaceutical laboratories owing to its abundance, low cost, non-toxicity, and nonflammability. However, due to CO2 inherent thermodynamic stability and kinetic inertness, it is difficult to convert CO2 to value-added chemicals under mild conditions. In order to overcome such barriers, numerous useful synthetic methodologies by strategically using highly active catalysts have been developed for the incorporation of CO2 to organic compounds. Transition metal-free compounds are proved to be promising efficacious catalysts able to activate CO2 molecule for efficient transformation of CO2 on the basis of mechanistic understanding at the molecular level. This chapter features recent advances at methodologies for catalytic transformation of CO2 promoted by organocatalysts (e.g., N-heterocyclic carbenes, frustrated Lewis pairs and superbases), ionic liquids, and main group metal to produce value-added chemicals such as linear or cyclic carbonates, quinazoline-2,4(1H,3H)-diones, alkylidene cyclic carbonates, amino acids, and so on.
6. Mei-Yan Wang, Ran Ma, Liang-Nian He*, Polyoxometalate-based ionic liquids-promoted CO2 conversion, Science China Chemistry, 2016, 59, 507-516. [link]

Abstract: Polyoxometalates(POMs) are a class of molecular metal oxides, showing numerous applications in various chemical processes due to their unique acid/base and redox features. By adjusting the tunable molecular structures of the anions and counter cations, plenty of POM-based ionic liquids(POM-based ILs) have been fabricated to be used in various fields, such as catalysis, structural chemistry and material science. As a class of excellent catalysts, POM-based ILs have shown advantages in the emerging field of CO2 utilization such as CO2 capture, cycloaddition of CO2 to epoxides, and reduction of CO2, owing to the efficient activation of CO2 by POM anions. This review summarizes recent advances in the catalysis of POM-based ILs, and particularly highlights the areas that are related to CO2 conversion.
7. Xiao-Fang Liu, Qing-Wen Song, Shuai Zhang, Liang-Nian He*, Hydrogen bonding-inspired organocatalysts for CO2 fixation with epoxides to cyclic carbonates, Catal. Today, 2016, 263, 69-74. [link]
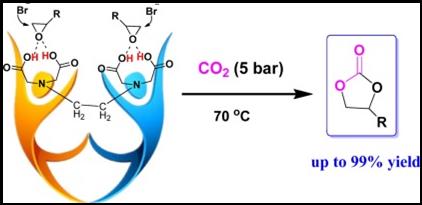
Abstract: Carboxyl-containing organocatalysts, e.g. EDTA (ethylenediaminetetraacetic acid) in combination with nucleophilic halide such as nBu4NBr were demonstrated to be efficient catalyst systems for the synthesis of cyclic carbonates from CO2 and a broad range of epoxides in excellent yield and selectivity. Thanks to synergistic effects of carboxylic groups and bromide anion, the cycloaddition reaction proceeded smoothly at 5 bar CO2 under mild reaction conditions. Interaction of carboxylic groups in EDTA with the epoxide via hydrogen bonding presumably facilitates the ring-opening of the epoxide by the nucleophile e.g. bromide. In particular, multiple carboxylic groups in one molecule i.e. EDTA could more effectively activate the epoxide and stabilize the alkoxide intermediate through multi-site hydrogen bonding in comparison with monocarboxylic acid. Moreover, the carboxylic acid like EDTA used in this study represents a cheap, commercially available, environmentally benign, metal-free catalyst for CO2 conversion. Thus, this catalytic protocol could have potential application for catalytic fixation of CO2 into value-added chemicals.
8. Zhang Shuai, Li Xuedong, He Liang-Nian*, Reductive Carboxylation of Unsaturated Hydrocarbons with Carbon Dioxide. Acta Chim. Sinica, 2016, 74(1): 17-23. [link]

Abstract: Transition metal-catalyzed reductive carboxylation of unsaturated hydrocarbons with CO2 is a promising and potential strategy, offering an excellent alternative access to carboxylic acids/acrylic acids. The active transition metal species could react with unsaturated hydrocarbons and CO2 to generate the stable metallalactones or carboxylic salts. The transmetalation between reductants and metallalactones/carboxylic salts regenerates the active catalytic species. As a result, the reductive carboxylation is able to run in a catalytic mode rather than stoichiometric version. Organometal species, silanes/boranes, metal powder, methanol and hydrogens have been developed as reducing reagents in reductive carboxylation with CO2. In this perspective, the latest advances on the transition metal-catalyzed reductive carboxylation are summarized, with particular focus on the application of reductants and related reaction mechanism at a molecular level.
9. Xi Liu, Shuai Zhang, Qing-Wen Song, Xiao-Fang Liu, Ran Ma and Liang-Nian He*. Cooperative calcium-based catalysis with 1,8-diazabicyclo[5.4.0]-undec-7-ene for the cycloaddition of epoxides with CO2 at atmospheric pressure, Green Chem., 2016, 18, 2871-2876. [link]

Abstract: A dual activation catalytic system consisting of CaBr2 and 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) was developed for efficient fixation of CO2 with epoxides to cyclic carbonates. Such dual catalysis renders the reaction to perform smoothly at atmospheric CO2 pressure, presumably due to simultaneous activation of CO2 by DBU and epoxide by CaBr2. In addition, the activation role of CaBr2 was also studied by density functional theory (DFT) calculation. A plausible mechanism involving the DBU-CO2 adduct assisted ring opening path and bromide anion-promoted ring opening path is proposed, in combination with the activation of epoxide by calcium cation. This process represents a simple, cost-effective and biocompatible route to cyclic carbonates from CO2 under mild condition, especially at atmospheric CO2 pressure.
10. Ran Ma, Yue-Biao Zhou, Liang-Nian He*, Carbon dioxide promoted reductive amination of aldehydes in water mediated by iron powder and catalytic palladium on activated carbon, Catal. Today, 2016, 274, 35-39. [link]
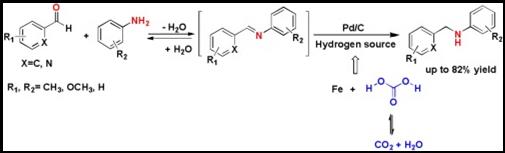
Abstract: A mixture of iron powder and catalytic palladium on activated carbon has been developed for reductive amination of various aromatic aldehydes, including 2-pyridinecarboxaldehyde, in water under CO2 atmosphere. The reversible reaction of CO2 with water could form carbonic acid and hydrogen transfer from water to Pd(0) took place with the presence of iron powder, leading to formation of high-active Pd hydrides for the reductive amination process. On the other hand, the reaction system could be inherently neutralized by ready removal of CO2, thus resulting in facile post-processing.
11. Xuedong Li, Xiandong Lang, Qingwen Song, Yakun Guo, Liang-Nian He*, Cu(I)-Catalyzed Three-component Reaction of Propargylic Alcohol, Secondary Amines and Atmospheric CO2, Chin. J. Org. Chem. 2016, 36, 744-751. [link]

Abstract: β-Oxopropylcarbamates constitute an important kind of organic compounds, owing to the extensive applications in agrochemicals, pharmaceuticals, organic synthesis, and protection of amino group. In this article, an efficient and atom-economical Cu(I) catalyzed three-component reaction of propargylic alcohols, secondary amines and CO2 has been developed under atmospheric pressure, affording various β-oxopropylcarbamates in high yields with high selectivity by controlling the concentration of O2. This protocol avoids the use of high pressure of CO2 and provides an extremely simple way to access the synthetically useful β-oxopropylcarbamates.

12. Xian-Dong Lang, Yi-Chen Yu, Zheng-Ming Li, Liang-Nian He*, Protic ionic liquids-promoted efficient synthesis of quinazolines from 2-aminobenzonitriles and CO2 at ambient conditions, J. CO2 Util., 2016, 15, 115-122. [link]

Abstract: Despite recent renaissance of CO2 chemistry, transformation of CO2 at ambient conditions remains still of great challenge. In this work, an easily prepared protic ionic liquid e.g. 1,1,3,3-tetramethylguanidinium imidazolide [HTMG][Im] was developed as highly efficient, recyclable and bifunctional catalyst for the carboxylative cyclization of 2-aminobenzonitriles with CO2 at atmospheric pressure and room temperature. The catalytic protocol was found to be applicable to various 2-aminobenzonitriles bearing electron-withdrawing or electron-donating substituents, affording the corresponding quinazoline-2,4(1H,3H)-diones in moderate to excellent yields. In addition, the catalyst could be conveniently recovered and reused for five cycles with almost consistent activity. Consequently, this process represents an alternative approach for the efficient and greener chemical fixation of CO2 to afford valuable heterocycles.
13. Zhen-Feng Diao, Zhi-Hua Zhou, Chun-Xiang Guo, Bing Yu and Liang-Nian He*, Propylene oxide as a dehydrating agent: potassium carbonate-catalyzed carboxylative cyclization of propylene glycol with CO2 in a polyethylene glycol/CO2 biphasic system, RSC Adv., 2016, 6(38), 32400-32404. [link]

Abstract: The synthesis of propylene carbonate (PC) from 1,2-propylene glycol (PG) and CO2 was smoothly performed in a PEG800 (polyethylene glycol)/CO2 biphasic system with K2CO3 as a catalyst and propylene oxide (PO) as a dehydrating agent. In the reaction of PG with CO2, PO presumably removes the water produced, and simultaneously generates more PG, both of which shift the thermodynamic control process and thus accelerate the PC synthesis. The PC yield directly from PG and CO2 reached 78% under relatively mild reaction conditions (4 MPa, 120 °C, 10 h). Notably, no additional by-product was detected in this process, resulting in economic benefits and the ease of workup procedure.
14. Xian-Dong Lang, Liang-Nian He*, Green Catalytic Process for Cyclic Carbonate Synthesis from Carbon Dioxide under Mild Conditions, Chem. Rec. 2016, 16, 1337-1352. [link]
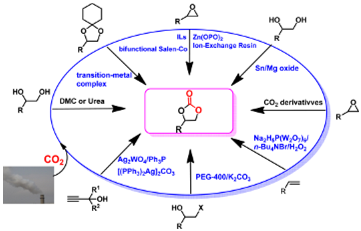
Abstract: As a renewable and abundant C1 resource possessing multiple attractive characteristics, such as low cost, nontoxicity, non-flammability, and easy accessibility, CO2 conversion into value-added chemicals and fuels can contribute to green chemistry and sustainable development. Since CO2 is a thermodynamically inert molecule, the activation of CO2 is pivotal for its effective conversion. In this regard, the formation of a transition-metal CO2 complex through direct coordination is one of the most powerful ways to induce the inert CO2 molecule to undergo chemical reactions. To date, numerous processes have been developed for efficient synthesis of cyclic carbonates from CO2. On the basis of mechanistic understanding, we have developed efficient metal catalysts and green processes, including heterogeneous catalysis, and metal-free systems, such as ionic liquids, for cyclic carbonate synthesis. The big challenge is to develop catalysts that promote the reaction under low pressure (preferably at 1 bar). In this context, bifunctional catalysis is capable of synergistic activation of both the substrate and CO2 molecule, and thus, could render CO2 conversion smoothly under mild conditions. Alternatively, converting CO2 derivatives, that is, the captured CO2 as an activated species, would more easily take place at low pressure in comparison with gaseous CO2. The aim of this Personal Account is to summarize versatile catalytic processes for cyclic carbonate synthesis from CO2, including epoxide/CO2 coupling reaction, carboxylation of 1,2-diol with CO2, oxidative cyclization of olefins with CO2, condensation of vicinal halohydrin with CO2, carboxylative cyclization of propargyl alcohols with CO2, and conversion of the CO2 derivatives.
15. Xian-Dong Lang, Yi-Chen Yu, Liang-Nian He*, Zn-salen complexes with multiple hydrogen bonding donor and protic ammonium bromide: bifunctional catalysts for CO2 fixation with epoxides at atmospheric pressure, J Mol Catal A: Chem. 2016, 420, 208-215. [link]

Abstract: Bifunctional Zn-salen complexes with multiple hydrogen bonding donors and protic ammonium bromides were developed as efficient catalysts for the formation of 4-phenyl-1,3-dioxolan-2-one from styrene oxide and CO2. Owing to synergistic effects of multiple sites on epoxide activation (Lewis acidic zinc, phenolic hydroxyl group and protonated tertiary ammonium able to activate epoxide and bromide anion acting as a nucleophile), the easily-synthesized Zn-salen complexes showed outstanding performance at mild reaction conditions (CO2 0.1 MPa, 100 °C) using only 0.3 mol% catalyst amount. Besides, this protocol is applicable to a variety of epoxides including internal epoxide, giving the corresponding cyclic carbonates in good to excellent yields. Notably, 200 mmol-scale experiment was successfully conducted and TON of 49288 ± 13 and TOF of 39473 ± 8 h−1 were achieved.
16. Hang Xu, Xiao-Fang Liu, Chun-Shuai Cao, Bin Zhao*, Peng Cheng and Liang-Nian He*, A Porous Metal–Organic Framework Assembled by [Cu30] Nanocages: Serving as Recyclable Catalysts for CO2 Fixation with Aziridines. Advanced Science, 2016, 3(11), 1600048. [link]
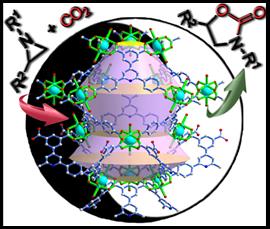
Abstract: Based on a novel ligand 5-(2,6-bis(4-carboxyphenyl)pyridin-4-yl)isophthalic acid (H4BCP) with large skeletons, a unique porous framework {[Cu2(BCP)(H2O)2]·3DMF}n (1) assembled by nano-sized and censer-like [Cu30] cages is successfully obtained and structurally characterized. In 1, the large 1D channel in frameworks and window size in the nanocages can enrich methylene blue and capture CO2, exhibiting the promising applications in environmental protection. More importantly, the explorations on the cycloaddition reaction of CO2 and aziridines with various substituents suggest that 1 can serve as an efficient heterogeneous catalyst for CO2 conversion with aziridines in a solvent-free system, which can be reused at least ten times without any obvious loss in catalytic activity. This is the first example of metal–organic framework (MOF)-based catalysts in converting CO2 into high-value oxazolidinones through activating aziridines and CO2, further extending the applications of MOFs materials in catalysis.
17. Zhi-Hua Zhou, Qing-Wen Song, Jia-Ning Xie, Ran Ma, Liang-Nian He*, Silver(I)-Catalyzed Three-Component Reaction of Propargylic Alcohols, CO2 and Monohydric Alcohols: Thermodynamically Feasible Access to β-Oxopropyl Carbonates. Chem. Asian J., 2016, 11, 2065-2071. [link]

Abstract: Silver(I)-catalyzed three-component reaction of propargylic alcohols, CO2, and monohydric alcohols was successfully developed for the synthesis of β-oxopropyl carbonates. As such, a series of β-oxopropyl carbonates were exclusively produced in excellent yields (up to 98%) even under atmospheric pressure of CO2. Silver catalyst in this study works efficiently for both the carboxylative cyclization of propargylic alcohols with CO2, and subsequent transesterification of α-alkylidene cyclic carbonates with monohydric alcohols, thus renders this tandem process performing smoothly under mild conditions. The present work provides a versatile and thermodynamically favorable approach to dissymmetric dialkyl carbonates.

18. Qing-Wen Song, Zhi-Hua Zhou, Mei-Yan Wang, Kan Zhang, Ping Liu, Jia-Yao Xun and Liang-Nian He*, Thermodynamically Favorable Synthesis of 2-Oxazolidinones through Silver-Catalyzed Reaction of Propargylic Alcohols, CO2, and 2-Aminoethanols, ChemSusChem, 2016, 9(16), 2054-2058. [link]
Abstract: Three in a pot: A thermodynamically feasible pathway for CO2 conversion is successfully performed to concurrently synthesize 2-oxazolidinones and α-hydroxyl ketones through a three-component reaction of propargylic alcohols, CO2, and 2-aminoalcohols. As a consequence, the thermodynamic limitation for the condensation reaction of 2-aminoalcohols and CO2 is circumvented by avoiding the dehydration step.
19. Jia-Ning Xie, Zhen-Feng Diao, Chang Qiao, Ran Ma and Liang-Nian He*, One-pot stepwise synthesis of cyclic carbonates directly from olefins with CO2 promoted by K2S2O8/NaBr, J. CO2 Util., 2016, 16, 313-317. [link]

Abstract: Efficient synthesis of cyclic carbonates from olefins using K2S2O8 as oxidant and NaBr as “bromination” reagent has been developed through a reaction sequence involving hydroxybromination of olefins and subsequent carboxylation of the bromohydrin in situ generated with CO2. This process provides a novel and convenient access to cyclic carbonates in a one-pot stepwise fashion. Representative olefins with different functional groups could react smoothly under relatively mild (3 MPa, 60 °C) and transition metal-free conditions to afford various cyclic carbonates.
20. Xiao-Fang Liu, Ran Ma, Chang Qiao, Han Cao, Liang-Nian He*, Fluoride-Catalyzed Methylation of Amines by Reductive Functionalization of CO2 with Hydrosilanes. Chem. Eur. J., 2016, 22(46), 16489-16493. [link]
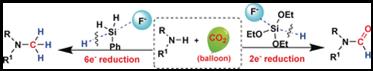
Abstract: An effective and inexpensive organocatalyst tetrabutylammonium fluoride (TBAF) was developed for the reductive functionalization of CO2 with amines to selectively afford formamides or methylamines by employing hydrosilanes. Hydrosilanes with different substituents show discriminatory reducing activity. Thus, the formation of formamides and further reduction products, that is, methylamines could be controlled by elegantly tuning hydrosilane types. Formamides were obtained exclusively under an atmospheric pressure of CO2 with triethoxysilane. Using phenylsilane as a reductant, methylamines were attained with up to 99 % yield at 50 °C coupled to a complete deoxygenation of CO2. The crucial intermediate silyl formate in the formylation step was identified and thereby a tentative mechanism involving the fluoride-promoted hydride transfer from the hydrosilane to CO2/formamide was proposed. Striking features of this metal-free protocol are formylation and methylation of amines by reductive functionalization of CO2 with hydrosilanes and mild reaction conditions.

21. Qing-Wen Song, Liang-Nian He*, Heterocyclic Synthesis Through C-N Bond Formation with Carbon Dioxide in Chemistry Beyond Chlorine, pp 435-453. [link]
22. Mei-Yan Wang, Hai-Bo Wang, Qiang-Hao Qu, Liang-Nian He*, Industrial Production of Dimethyl Carbonate from CO2 in China in Chemistry Beyond Chlorine, pp 387-411. [link]