论文发表
4. Xiao-Rong Wen, Shan-Shan Chen, Wen-Jun Xie, Hong-Ru Li, Liang-Nian He*, Cavity-confined Cu2O nanoreactors for efficient CO2 electroreduction to ethylene. J Catal, 2026, 455, 116710. [Link]
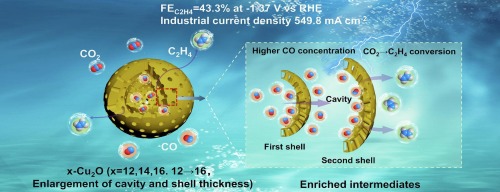
Abstract: Electrocatalytic carbon dioxide reduction (eCO2R) to ethylene (C2H4) offers a promising pathway toward carbon–neutral cycles and sustainable chemical synthesis. In eCO2R to C2H4, copper-based electrocatalysts, particularly Cu2O, have been widely used. Nevertheless, product selectivity to C2H4 is still quite limited due to the insufficient enrichment of key intermediates and slow C–C coupling kinetics. Herein, the double-shelled hollow mesoporous Cu2O was fabricated through the soft-templating method. And the cavity size and pore architecture of the resulting Cu2O catalysts could be precisely tailored by adjusting the alkyl chain length of the surfactant templates alkyltrimethylammonium bromides. When applied to eCO2R, the as-prepared Cu2O material with tetradecyltrimethylammonium bromide (TTAB) as template exhibited a remarkable Faradaic efficiency of 43.3 ± 0.8% for C2H4 at an industrial-level current density of 549.8 mA cm−2. Experimental and theoretical investigations reveal that its high activity and selectivity toward C2H4 stem from the suitable cavity configuration, which enriches key *CO intermediates and promotes their dimerization via a spatial confinement effect. This study provides valuable insights into the architectural design of efficient catalysts for CO2-to-C2H4 conversion.
3. Li-Feng Xu, Wen-Jun Xie, Shan-Shan Chen, Alexander O. Terent'ev,c Hong-Ru Li* and Liang-Nian He*. Direct synthesis of cyclic carbonates from olefins and CO2 via ionic liquid catalysis with mutually promoting bifunctional groups. Green Chem.,2026, DOI: 10.1039/D5GC06239J. [Link]
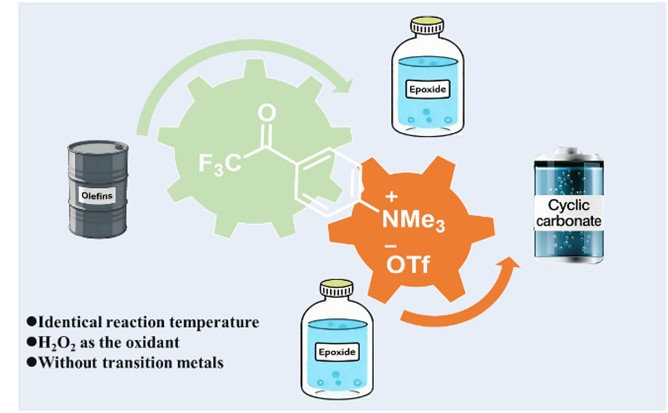
Abstract: The oxidative carboxylation of olefins is a thermodynamically and economically favorable strategy for the synthesis of cyclic carbonates. As a common oxidant, aqueous hydrogen peroxide solution serves as a cost-effective and green chemical. Herein, we develop a bifunctional ionic liquid, which has both the trifluoroacetyl group as the epoxidation reactive site and quaternary ammonium salt for promoting the cycloaddition reaction. The two active sites work synergistically through the electronic effect, thereby rendering two subsequent steps compatible and allowing the target reaction to proceed efficiently and selectively accordingly. The introduction of quaternary ammonium salt enhances the selectivity of epoxides at 80 °C. The trifluoroacetyl group augments the activating capability of the quaternary ammonium site toward epoxides and ultimately allows the cycloaddition reaction to proceed efficiently at a lower temperature. With hydrogen peroxide as the oxidant, the bifunctional ionic liquid catalyst facilitates a two-step process for the synthesis of cyclic carbonates from olefins and CO2 in high yield, reaching up to 90%, in the absence of any transition metal. This work provides a novel, green, and sustainable strategy for the oxidative carboxylation of alkenes to synthesize cyclic carbonates.
2. Lada A. Zaikina, Mikhail M. Doronin, Oleg O. Segida, Olga M. Mulina, Igor B. Krylov, Liang-Nian He and Alexander O. Terent'ev*.Synthesis of thiazoles from vinyl azides and xanthates under the action of an Mn(III)-oxidant. Org. Biomol. Chem., 2026, 24, 136. [Link]
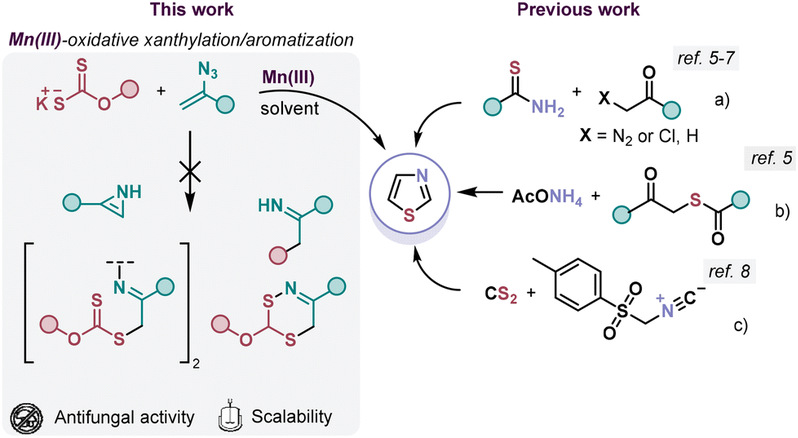
Abstract: Reaction of xanthates and vinyl azides under the action of Mn(OAc)3 results in the formation of alkoxy thiazoles. In this transformation, potassium xanthate undergoes Mn-mediated oxidation, generating the corresponding xanthyl radical. The latter interacts with the double bond of the vinyl azide, and after N2 elimination, a β-xanthylated iminyl radical is formed. The quenching of the iminyl radical by an Mn(II)-ion with subsequent cyclization into a 5-membered ring, an unexpected elimination of a sulfur-containing fragment and aromatization lead to thiazoles. It is important to mention that cyclization with the formation of a 6-membered ring is not observed in the disclosed process. The obtained thiazoles demonstrate antifungal activity surpassing that of commercially available fungicides.
1. Valerio D’ Elia*, Pichaya Pattanasattayavong, Liang-Nian He*. 4-Aminopyridines as Versatile “Noninterfering” Allies for CO2 Fixation. ChemCatChem 2026, 18, e01150. [Link]
Abstract: Organic superbases are a family of compounds endowed with high nucleophilicity and basicity. Several powerful nucleophiles such as DBU (1,8-diazabicyclo[5.4.0]undec-7-ene), TBD (1,5,7-triazabicyclo[4.4.0]dec-5-ene), or DMAP (4-dimethylaminopyridine) are involved in CO2 conversion but their catalytic roles may differ from a mechanistic standpoint. In this work, we show the versatile application of 4-aminopyridines in CO2 fixation leading to products of CO2 reduction as well as cyclic carbonates and fine chemicals. In such cases, 4-aminopyridines serve not just as organocatalysts, but as recurring motifs performing as bases, structural components, ligands for electronic modulation of metals and full-fledged catalytic components. Such roles are highlighted herein with an eye to the understanding of mechanistic aspects and the interaction between 4-aminopyridines and CO2 through the discussion of several catalytic studies.
专利申请和授权
1. 陈珊珊,许立锋,李红茹,何良年,一种双功能离子液体催化烯烃氧化羧化合成环状碳酸酯的方法 ,专利号:ZL 2025 1 1707838.9