论文发表
16. An-Guo Wu, Jie Ding, Lan Zhao, Hong-Ru Li, and Liang-Nian He*. Reductive Transformation of CO2 to Organic Compounds. Chem. Rec. 2024, 24(12), e202400164. [Link]

Abstract: Carbon dioxide is a major greenhouse gas and a safe, abundant, easily accessible, and renewable C1 resource that can be chemically converted into high value-added chemicals, fuels and materials. The preparation of urea, organic carbonates, salicylic acid, etc. from CO2 through non-reduction conversion has been used in industrial production, while CO2 reduction transformation has become a research hotspot in recent years due to its involvement in energy to rage and product diversification. Designing suitable catalysts to achieve efficient and selective conversion of CO2 is crucial due to its thermodynamic stability and kinetic inertness. From this perspective, the redistribution of charges within CO2 molecules through the interaction of Lewis acid/base or metal complexes with CO2, or the forced transfer of electrons to CO2 through photo- or electrocatalysis, is a commonly used effective way to activate CO2. Based on understanding of the activation/reaction mechanism on a molecular level, we have developed metal complexes, metal salts, inorganic/organic salts, ionic liquids, as well as nitrogen rich and porous materials as efficient catalysts for CO2 reductive conversions. The goal of this personal account is to summarize the catalytic processes of CO2 reductive conversion that have been developed in the past 7 years: 1) For the reductive functionalization of CO2, the major challenges in accurately adjusting reaction parameters (such as pressure) to achieve high catalytic efficiency and the product selectivity; 2) For photocatalytic or electrocatalytic reduction of CO2, how to suppress competitive hydrogen evolution reactions and improve catalyst stability are key points that requires continuous attention.

Abstract: Herein, we report an electrochemical protocol for the dicarboxylation of aryl alkynes using CO2. With a graphite rod as the cathode and Al as the sacrificial anode, a series of valuable butenedioic acids are obtained in moderate to excellent yields with an E/Z ratio up to 50:1. This method features high E-selectivity, high step and atom economy, easy scalability, and a nice substrate scope, which renders it appealing for promising applications in organic synthesis and materials chemistry.
14. Hai-Yang Hu, Wen-Jun Xie, Hong-Ru Li*, Liang-Nian He*, Ether chain-modified Alkanolguanidine for CO2 capture and subsequent conversion, Carbon Capture Sci. Technol., 2024, 13, 100284. [Link]

Abstract: Capturing CO2 and converting it into valuable chemicals has attracted considerable attention in recent years. Herein, a kind of ether chain-modified alkanolguanidines (ECMAs) was designed and synthesized as a dual functional reagent for carbon dioxide capture and conversion. Due to the presence of basic sites and CO2-philic ether chains, these ECMAs demonstrated almost equimolar CO2 capture at room temperature and atmospheric pressure through the synergy of physical and chemical absorption. Even for diluted CO2 (15 % CO2), 0.7 mol CO2 per mole capture reagent can still be achieved, showing their potential application in post-combustion capture. The synthesized ECMAs can also serve as catalyst in the cycloaddition reaction of CO2 with various epoxides, affording 75–99 % yield of corresponding cyclic carbonates under 3 MPa CO2 with tetrabutylammonium iodide (TBAI) as co-catalyst. Moreover, these ECMAs can be applied to the integrated CO2 capture and conversion, in which the ECMAs can react with CO2, forming the alkyl carbonate zwitterion as active CO2 species in the capture step. And in the subsequent cycloaddition reaction with propylene oxide, 52 % yield of propylene carbonate was obtained.
13. Dmitri I. Fomenkov, Roman A. Budekhin, Peter S. Radulov, Alexander I. Fomenkov, Liang-Nian He, Ivan A. Yaremenko*, Alexander O. Terent’ev* C═N Bond Ozonolysis and β-Scission: A Breakthrough Approach to the Synthesis of ω-Functionalized Compounds from Carbonyl Derivatives. Org. Lett. 2024, 26, 8095-8099. [Link]

Abstract: This work discloses a two-step, one-pot approach to ω-functionalized esters via cleavage of the alicyclic fragment of cycloalkanone semicarbazones. This approach is based on a combination of the synthesis of various alkoxyhydroperoxides via cycloalkanone semicarbazone ozonolysis and in situ interaction of these peroxides with transition metal salts, leading to cleavage of the aliphatic cycle and subsequent ω-functionalized ester formation. A broad series of ω-halogen or pseudohalogen esters have been successfully synthesized in yields ranging from 23 to 73% per starting semicarbazone. A major advantage of the approach is the ability to use different cycloalkanone semicarbazones, including those with large cycles and substituents in them. The possibility of carrying out ozonolysis in the presence of various alcohols makes it possible to obtain the corresponding esters of ω-substituted carboxylic acids.
12. An-Guo Wu, Jie Ding, Lan Zhao, Hong-Ru Li, Liang-Nian He*, Hydroformylation of Olefins with CO2/H2 and Hydrosilane by Copper/Cobalt Tandem Catalysis. ChemSusChem 2024, 17, e202400608. [Link]

Abstract: A Cu/Co tandem catalysis protocol was developed to conduct the hydroformylation of olefins using CO2/H2 and PMHS (polymethylhydrosiloxane) as a readily available and environmentally friendly hydride source. This methodology was performed via a two-step approach consisting of the copper-catalyzed reduction of CO2 by hydrosilane and subsequent cobalt-promoted hydroformylation with H2 and the in situ formed CO. The optimized triphos oxide ligand, which presumably facilitates the migratory insertion of CO gives moderate to excellent yields for both terminal and internal alkenes. This earth-abundant metal catalysis provides a reliable and efficient way to afford useful aldehydes in industry using silicon by-product PMHS as hydrogen source and renewable CO2 as carbonyl source.
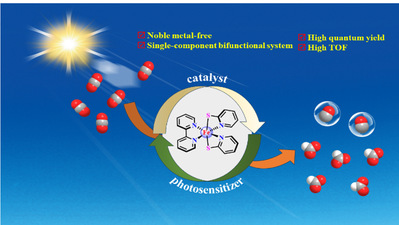
Abstract: Designing earth-abundant metal complexes as efficient molecular photocatalysts for visible light-driven CO2 reduction is a key challenge in artificial photosynthesis. Here, we demonstrated the first example of a mononuclear iron pyridine-thiolate complex that functions both as a photosensitizer and catalyst for CO2 reduction. This single-component bifunctional molecular photocatalyst efficiently reduced CO2 to formate and CO with a total turnover number (TON) of 46 and turnover frequency (TOF) of 11.5 h-1 in 4 h under visible light irradiation. Notably, the quantum yield was determined to be 8.4% for the generation of formate and CO at 400 nm. Quenching experiments indicate that high photocatalytic activity is mainly attributed to the rapid intramolecular quenching protocol. The mechanism investigation by DFT calculation and electrochemical studies revealed that the protonation of Febpy(pyS)2 is indispensable step for photocatalytic CO2 reduction.
10. Liqi Qiu, Yuqing Fu, Zhenzhen Yang*, Anna C. Johnson, Chi-Linh Do-Thanh, Bishnu P. Thapaliya, Shannon M. Mahurin, Liang-Nian He, De-en Jiang*, Sheng Dai*, Surpassing the Performance of Phenolate-derived Ionic Liquids in CO2 Chemisorption by Harnessing the Robust Nature of Pyrazolonates. ChemSusChem 2024, 17 (6), e202301329. [Link]

Abstract: Superbase-derived ionic liquids (SILs) are promising sorbents to tackle the carbon challenge featured by tunable interaction strength with CO2 via structural engineering, particularly the oxygenate-derived counterparts (e. g., phenolate). However, for the widely deployed phenolate-derived SILs, unsolved stability issues severely limited their applications leading to unfavorable and diminished CO2 chemisorption performance caused by ylide formation-involved side reactions and the phenolate-quinone transformation via auto-oxidation. In this work, robust pyrazolonate-derived SILs possessing anti-oxidation nature were developed by introducing aza-fused rings in the oxygenate-derived anions, which delivered promising and tunable CO2 uptake capacity surpassing the phenolate-based SIL via a carbonate formation pathway (O−C bond formation), as illustrated by detailed spectroscopy studies. Further theoretical calculations and experimental comparisons demonstrated the more favorable reaction enthalpy and improved anti-oxidation properties of the pyrazolonate-derived SILs compared with phenolate anions. The achievements being made in this work provides a promising approach to achieve efficient carbon capture by combining the benefits of strong interaction strength of oxygenate species with CO2 and the stability improvement enabled by aza-fused rings introduction.
9. Jin-Mei Chen, Wen-Jun Xie, Zhi-Wen Yang, Liang-Nian He*, Molecular Engineering of Copper Phthalocyanine for CO2 Electroreduction to Methane. ChemSusChem 2024, 17 (6), e202301634. [Link]
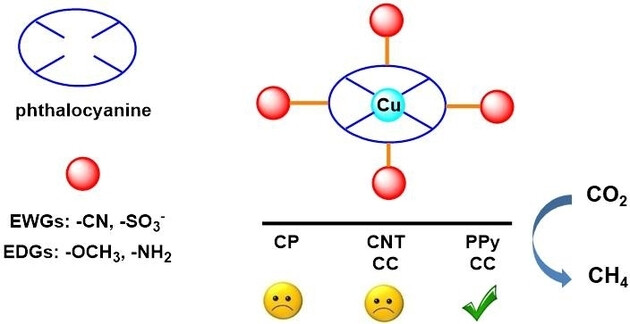
Abstract: Efficient electrochemical CO2 reduction reaction (ECO2RR) to multi-electron reductive products remains a great challenge. Herein, molecular engineering of copper phthalocyanines (CuPc) was explored by modifying electron-withdrawing groups (EWGs) (cyano, sulfonate anion) and electron-donating groups (EDGs) (methoxy, amino) to CuPc, then supporting onto carbon paper or carbon cloth by means of droplet coating, loading with carbon nanotubes and coating in polypyrrole (PPy). The results showed that the PPy-coated CuPc effectively catalysed ECO2RR to CH4. Interestingly, experimental results and DFT calculations indicated EWGs markedly improved the selectivity of methane for the reason that the introduction of EWGs reduces electron density of catalytic active center, resulting in a positive move to initial reduction potential. Otherwise, the modification of EDGs significantly reduces the selectivity towards methane. This electronic effect and heterogenization of CuPc are facile and effective molecular engineering, benefitting the preparation of electrocatalysts for further reduction of CO2.
8. Lan Zhao, Liang-Nian He*, The Chlorine Evolution Reaction Promoted by Organocatalysts with Amide Functional Groups. Sci. China Chem. 2024, 67, 749-750. [Link]
7. Lan Zhao, Hai-Yang Hu, An-Guo Wu, Alexander O. Terent’ev, Liang-Nian He*, Hong-Ru Li*. CO2 capture and in-situ conversion to organic molecules. J. CO2 Util. 2024, 82, 102753. [Link]

To address the CO2 accumulation in atmosphere, various initiatives have been proposed, among which CO2 capture and utilization (CCU) is regarded as an appealing strategy to reconcile carbon emission and resource utilization. Especially, integrated CO2 capture and utilization (ICCU), i.e. performing CO2 capture and in-situ conversion can circumvent the energy-intensive CO2 desorption step and thus facilitate establishing step- and energy-efficient process, rendering the conversion at mild conditions particularly at low pressure due to substantial activation upon CO2 uptake. However, CO2 capture and in-situ conversion is not the simple add-up of these two processes. Its successful implementation relies on the harmonization of CO2 capture reagents, substrates and the corresponding catalysts. By far, tremendous efforts have been made in this field and a plethora of CO2 capture reagents including inorganic bases, organic bases, ionic liquids and carbonaceous materials have been utilized to capture CO2 and the conversion protocols such as hydrogenation, cycloaddition, carboxylative cyclization etc. have been explored for these captured CO2. As a result, the valuable products containing methanol, methane, carbonates, carbamates, oxazolidinones, ureas, and quinazolinone have been obtained from CO2 and more importantly, the CO2 chemistry theory is also enriched via investigating the structure and reactivity of the captured CO2 in various reactions. In this review, we summarize the progress on CO2 capture and in-situ conversion based on the reaction types and corresponding CO2 absorbents. It’s hoped that this review can shed light on the design of CO2 capture and in-situ conversion and inspire the further development of this field.
6. Alexander O. Ustyuzhanin,a Oleg V. Bityukov,a Pavel V. Sokolovskiy, a Valentina M. Merkulova,a Alexey I. Ilovaisky, Liang-Nian He, Vera A. Vil’* and Alexander O. Terent’ev*Electrochemical hydrocarboxylation of enol derivatives with CO2: access to b-acetoxycarboxylic acids. Chem. Commun. 2024, 60, 8099-8102. [Link]

Abstract: Electrochemical hydrocarboxylation of enol acetates with CO2 is developed. The disclosed process provides β-acetoxycarboxylic acids in 25–66% yields, in contrast to the electrolysis of ketones, silyl enol ethers and vinyl tosylates with CO2, which leads mainly to alcohols.
5. Ivan A. Yaremenko,* Dmitri l. Fomenkov, Roman A. Budekhin, Peter S. Radulov, Michael G. Medvedev.Nikolai V. Krivoshchapov, Liang-Nian He, Igor V. Alabugin,* and Alexander O. Terent'ev*, Interrupted Dance of Five Heteroatoms: Reinventing Ozonolysis to Make Geminal Alkoxyhydroperoxides from C═N Bonds. J. Org. Chem. 2024, 89, 5699-5714.[Link]
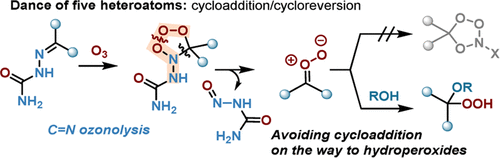
Abstact: Four heteroatoms dance in the cascade of four pericyclic reactions initiated by ozonolysis of C═N bonds. Switching from imines to semicarbazones introduces the fifth heteroatom that slows this dance, delays reaching the thermodynamically favorable escape path, and allows efficient interception of carbonyl oxides (Criegee intermediates, CIs) by an external nucleophile. The new three-component reaction of alcohols, ozone, and oximes/semicarbazones greatly facilitates synthetic access to monoperoxyacetals (alkoxyhydroperoxides).
4. Oleg V. Bityukov, Pavel Yu. Serdyuchenko, Vera A. Vil', Gennady I. Nikishin, Liang-Nian He, Alexander O. Terent'ev*, Co-Catalyzed Peroxidation of Cyclic β-Dicarbonyls. Eur. J. Org. Chem. 2024, 27, e202400078. [Link]
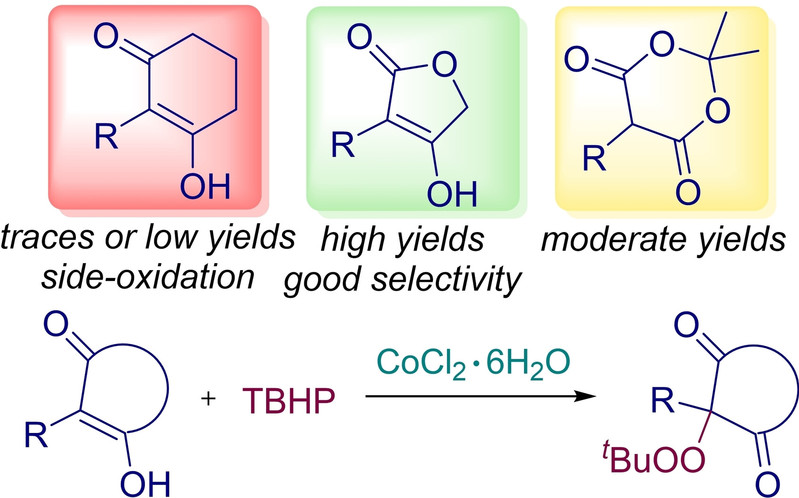
Abstract: A Co-catalyzed peroxidation of cyclic β-dicarbonyls (cyclic 1,3-diketones, 4-hydroxy-2(5H)-furanones and Meldrum's acids) with TBHP has been disclosed. A series of the alkylperoxy derivatives of 4-hydroxy-2(5H)-furanones and Meldrum's acids were synthesized in moderate to good yields (13–86 %). The functionalization of 4-hydroxy-2(5H)-furanones by the tBuOO group was performed with high selectivity in the presence of the cocktail of reactive oxidizing species, including metal and radical intermediates. The key species in the peroxidation process are probably the tert-butylperoxy radical or its Co(III) complex, which are generated from the Co(II)/TBHP system. Cycle cleavage, which would be expected for cyclic β-dicarbonyls based on previous reports, was observed to a large extent only for cyclic 1,3-diketones.
3. Meng-Ge Wei, Hong-Ru Li*, Liang-Nian He*, Synthesis of Dimethyl Carbonate via Transesterification of Ethylene Carbonate and Methanol over Mesoporous KF-loaded Mg-Fe Oxides, ChemPlusChem 2024, 89, e202300778. [Link]
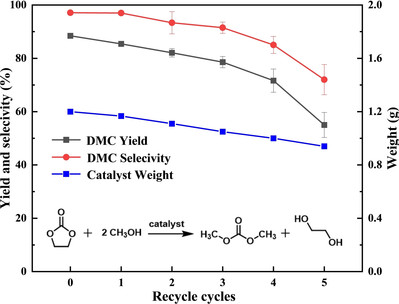
Abstract: A series of KF/Mg-Fe oxides were fabricated via the solid-state reaction between KF and Mg-Fe oxides. Especially, when 20 wt % KF was supported on the Mg-Fe bi-metal oxides and calcined at 400–600 °C, the solid material with more basic sites than the support itself was obtained. When applied as catalyst to dimethyl carbonate (DMC) synthesis through transesterification of ethylene carbonate (EC) and methanol, this material can afforded up to 88 % yield and 97 % selectivity toward DMC in 2 h under reflux conditions with the molar ratio of methanol to ethylene carbonate set at 8. It is worth noting that the catalyst was easily separated and reused, retaining at least 89 % catalytic activity during the first four recycles. Although an attenuated activity was still observed due to the inevitable filtration loss and dissolution, this solid base can still provide clues to the development recyclable catalyst in green synthesis of DMC.
2. Heng Li, Song Gao, Wei-Hang Xie, Liang-Nian He*, Hong-Ru Li*, Brønsted Acid-functionalized Silica-coated Magnetic Catalyst for the Preparation of Polyol Ricinoleates. Biomass Conv. Bioref. 2024, 14, 15061-15068. [Link]

Abstract: As a kind of non-edible vegetable oil, castor oil has attracted considerable interest in developing bio-based products due to its unique structure, among which polyol ricinoleates are considered as promising alternatives to mineral oil-based lubricants. In this article, a Brønsted acid-functionalized silica-coated magnetic catalyst (Fe3O4@SiO2-IM-SO3H) was developed as the efficient heterogeneous catalyst for the preparation of polyol ricinoleates via the esterification of ricinoleic acid with trimethylolpropane (TMP), neopentyl glycol (NPG), and pentaerythritol (PE), respectively. The structure and magnetic property of the catalyst were characterized by Fourier infrared spectroscopy (FT-IR), X-ray diffractometer technology (XRD), element analysis (CHN), and vibrating sample magnetometer (VSM). As a result, the sulfonic group was proposed to promote the esterification and the magnetism facilitated the catalyst separation. Notably, the yield of polyol ricinoleates attained successfully up to 99.5%, and the catalyst was recycled for at least five times without obvious activity reduction. This work about the utilization of magnetic nanoparticles supported acid catalysts in polyol ricinoleates preparation features clean and sustainability, providing an example of developing green products.
1. 许立锋, 武安国, 于芳羽, 李红茹*,何良年*,可再生能源驱动的CO2基环状碳酸酯合成研究进展. 有机化学,2024, 44, 3091-3105. [Link]

摘要:环状碳酸酯是重要的化工产品,在电池电解液、化妆品、油漆等领域具有广泛应用,同时还是有机合成的绿色溶剂和反应原料.环状碳酸酯是工业规模CO2转化的重要产品,主要通过环氧化物与CO2的环加成反应进行制备.在传统的热催化反应中,由于环氧化物活化开环具有较高的能垒,因而一般需要较高的反应温度.近年来,新材料的发展推动了环加成反应催化剂的创新,新的催化模式不断出现,尤其是光驱动和电驱动的环加成反应实现了温和条件下环状碳酸酯的制备.此外,电驱动的烯烃或邻二醇与CO2合成环状碳酸酯也取得了初步的研究成果.总结了光/电驱动的CO2合成环状碳酸酯的反应,旨在通过对材料设计和催化机理的介绍,为新型反应路径和催化材料的设计提供新思路.
专利申请和授权
1. 魏梦歌,李红茹,何良年,一种基于新型固体碱催化合成碳酸二甲酯的方法,申请号:202311798945.8
2. 武安国,李红茹,何良年,铜/钴配合物接力催化烯烃和二氧化碳氢甲酰化反应制备醛类化合物,申请号:202410461551.1
3. 胡海洋,谢汶均,李红茹,何良年,一种基于烷醇胍类吸收剂的CO2捕集与原位转化的方法,申请号:202411148567.3