1. Yu-Nong Li, Ran Ma, Liang-Nian He* and Zhen-Feng Diao. Homogeneous hydrogenation of carbon dioxide to methanol. Catal. Sci. Technol., 2014, 4(6), 1498-1512. [link]
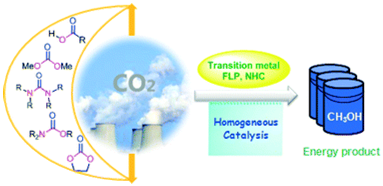
Abstract: Carbon dioxide, a greenhouse gas mainly from the consumption of fossil fuel, is regarded as an attractive feedstock in view of synthetic chemistry. Great efforts have been devoted to developing catalytical processes for converting CO2 into value-added compounds with reduced carbon footprint. Among versatile applications in organic synthesis, CO2 can serve as a promising raw material for fuel production including methanol, CH4, etc. ‘Coming from fuel and returning to fuel’ is an appealing objective in terms of sustainable development associated with circumventing energy shortage and CO2 issue. To date, metal complexes and organocatalysts for CO2 hydrogenation to both liquefied and gaseous fuels have been developed along with the reaction mechanistic insight. Understanding the interaction of active catalytic species with CO2 or hydrogen could account for development of efficient homogeneous catalysts. In this context, homogeneous catalytic hydrogenation of CO2 and its derivatives into fuel-related products is highlighted in this article in combination with mechanistic understanding on a molecular level.

2. Zhen-Zhen Yang, De-en Jiang, Xiang Zhu, Chengcheng Tian, Suree Brown, Chi-Linh Do-Thanh, Liang-Nian He* and Sheng Dai*. Coordination effect-regulated CO2 capture with an alkali metal onium salts/crown ether system. Green Chem., 2014, 16, 253-258. [link]
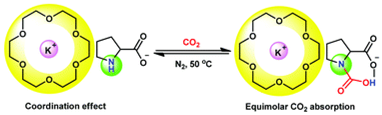
Abstract: A coordination effect was employed to realize equimolar CO2 absorption, adopting easily synthesized amino group containing absorbents (alkali metal onium salts). The essence of our strategy was to increase the steric hindrance of cations so as to enhance a carbamic acid pathway for CO2 capture. Our easily synthesized alkali metal amino acid salts or phenolates were coordinated with crown ethers, in which highly sterically hindered cations were obtained through a strong coordination effect of crown ethers with alkali metal cations. For example, a CO2 capacity of 0.99 was attained by potassium prolinate/18-crown-6, being characterized by NMR, FT-IR, and quantum chemistry calculations to go through a carbamic acid formation pathway. The captured CO2 can be stripped under very mild conditions (50 °C, N2). Thus, this protocol offers an alternative for the development of technological innovation towards efficient and low energy processes for carbon capture and sequestration.
3. Yu-Nong Li, Zhen-Feng Diao, Liang-Nian He*, and Zhen-Zhen Yang, Carbon capture with simultaneous activation and its subsequent transformation. Advances in Inorganic Chemistry, Vol 65, Chapter 9, Elsevier Science, 2014, in press (Book Chapter invited by the editor)
4. Qing-Wen Song, Bing Yu, Xue-Dong Li, Ran Ma, Zhen-Feng Diao, Rong-Guan Li, Wei Li* and Liang-Nian He*. Efficient chemical fixation of CO2 promoted by a bifunctional Ag2WO4/Ph3P system. Green Chem., 2014, 16, 1633-1638. [link]
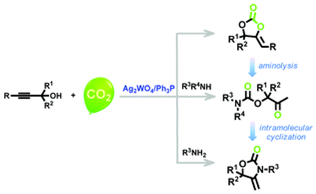
Abstract: An efficient heterogeneous silver-catalyzed reaction for construction of the α-methylene cyclic carbonate motif was developed through carboxylative assembly of propargyl alcohols and CO2. Such a CO2 fixation protocol proceeded smoothly with only 1 mol% of Ag2WO4 and 2 mol% of PPh3 as well as atmospheric CO2 at room temperature under solvent-free conditions, in an environmentally benign and low energy manner along with an easy operating procedure. Notably, up to 98% isolated yields of carbonates could be attained with exclusive chemo-selectivity. In addition, the dual activation capacity of Ag2WO4 towards both the propargylic substrate and CO2 is based on which cooperative catalytic mechanism by the silver cation and the tungstate anion is proposed. Recycling trials on carboxylative cyclization of propargyl alcohols and CO2 illustrate that the catalyst can be reused at least 4 times with retention of high catalytic activity and selectivity. Especially, it allows the direct and effective application in the one-pot synthesis of various oxazolidinones bearing exocyclic alkenes and carbamates in moderate to high yields upon the alternative introduction of primary or secondary amines.
5. Bing Yu, Chun-Xiang Guo, Chun-Lai Zhong, Zhen-Feng Diao, Liang-Nian He*. Metal-free chemoselective oxidation of sulfides by in situ generated Koser’s reagent in aqueous media. Tetrahedron Lett., 2014, 55, 1818-1821. [link]
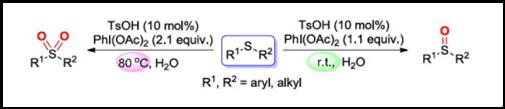
Abstract: Selective oxidation of sulfides was successfully performed by employing phenyliodine diacetate as oxidant with the catalysis of TsOH in aqueous solution under mild conditions. Sulfoxides were formed with 1.1 equiv. of PhI(OAc)2 at room temperature; whereas sulfones were obtained in the presence of 2.1 equiv. of PhI(OAc)2 at 80 °C under otherwise identical conditions. Notably, various sulfides were converted to corresponding sulfoxides or sulfones in good to high yields by this metal-free protocol.
6. Ran Ma, Liang-Nian He*, Qing-Wen Song, Yue-Biao Zhou and Kai-Xuan Liu, Efficient hydrogenation of imines over Fe and ZnO powder in self-neutralizing acidic CO2/H2O system. RSC Adv., 2014, 14, 11867-11871. [link]
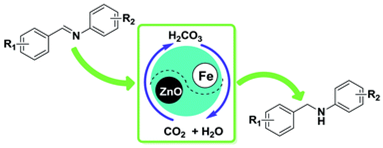
Abstract: An environmentally benign process was developed for the reduction of imines with iron and zinc oxide-promoted in the reversible CO2/H2O system without any acid additive or organic solvent. H2O is considered to serve as the terminal hydrogen source. The reaction system could be inherently neutralized by readily removal of CO2, thus resulting in facile post-processing and none waste disposal. The highly flexible and chemo-selective reduction protocol affords the corresponding amines with intact other reducible substituents in good to excellent yields.
7. Shuai Zhang, Yu-Nong Li, Ya-Wei Zhang, Liang-Nian He,* Bing Yu, Qing-Wen Song, Xian-Dong Lang, Equimolar Carbon Absorption by Potassium Phthalimide and In Situ Catalytic Conversion Under Mild Conditions, ChemSusChem, 2014, 7, 1484-1489. [link]
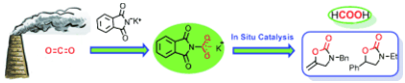
Abstract: In a fix: Potassium phthalimide is as an excellent absorbent for equimolar CO2capture with simultaneous activation. The in situ catalytic conversion of captured CO2 can be successfully converted into value-added chemicals and fuel-related products under mild conditions through a carbon capture and utilization pathway, rather than going through desorption process.
8. Bing Yu, Zhen-Feng Diao, An-Hua Liu, Xu Han, Bin Li, Liang-Nian He* and Xiang-Ming Liu, Selective Oxidation of Sulfides to Sulfoxides with tert-Butylnitrite as An Alternative Oxidant, Curr. Org. Synth., 2014, 11(1), 156-160. [link]

Abstract: Tert-butylnitrite was proved to be an efficient oxidant for the selective oxidation of sulfides to sulfoxides. The reaction was promoted by Fe(NO3)3·9H2O under mild conditions and various substrates were effectively converted into the corresponding sulfoxides in good yields and excellent selectivity.
9. Ya-Nan Zhao, Bing Yu, Zhen-Zhen Yang and Liang-Nian He*, Magnetic base catalysts for the chemical fixation of carbon dioxide to quinazoline-2,4(1H,3H)-diones, RSC Adv., 2014, 4, 28941-28946. [link]
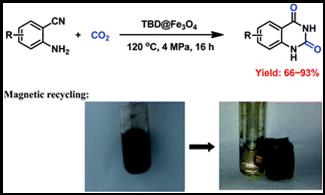
Abstract: TBD-functionalized Fe3O4 was proven to be an efficient and recyclable magnetic heterogeneous catalyst for the chemical fixation of CO2 with 2-aminobenzonitriles under mild conditions.
10. Shuai Zhang and Liang-Nian He*, Capture and Fixation of CO2 Promoted by Guanidine Derivatives, Aust. J. Chem. 2014, 67(7), 980-988. [link]
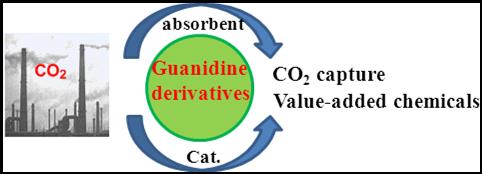
Abstract: Guanidine compounds and their derivatives can be developed as catalysts, additives, or promoters in organic synthesis due to their unique chemical properties, which have attracted much attention in the chemistry and catalysis communities. Particularly, the strong basicity and ease of structural modification allow them to offer wide applications in the field of CO2 capture and conversion. Guanidine compounds modified as ionic liquids or heterogeneous catalysts have also been developed for CO2 capture and conversion. In this context, the latest progress on CO2 capture using guanidine and their derivatives as absorbents with high capacity will be summarized. Furthermore, guanidine-catalyzed transformation of CO2 to a series of value-added chemicals with mechanistic consideration on a molecular level will be particularly elaborated in this article.
11. Ran Ma, Cheng-Bin Huang, An-Hua Liu, Xue-Dong Li and Liang-Nian He*, An in situ acidic carbon dioxide/glycol system for aerobic oxidative iodination of electron-rich aromatics catalyzed by Fe(NO3)3·9H2O. Catal. Sci. Technol., 2014, 4, 4308-4312. [link]
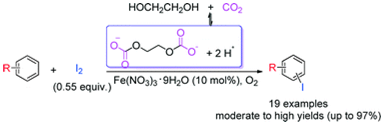
Abstract: An environmentally benign CO2/glycol reversible acidic system was developed for the iron(III)-catalyzed aerobic oxidative iodination of electron-rich aromatics without the need for any conventional acid additive or organic solvent. Notably, moderate to high isolated yields (up to 97%) of the aryl iodides were attained with comparable regioselectivity when ferric nitrate nonahydrate was used as the catalyst with molecular iodine under 1 MPa of CO2.
12. Zhen-Zhen Yang and Liang-Nian He*, Efficient CO2 capture by tertiary amine-functionalized ionic liquids through Li+-stabilized zwitterionic adduct formation, Beilstein J. Org. Chem., 2014, 10, 1959-1966. [link]
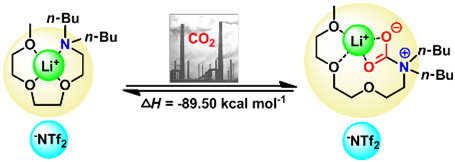
Abstract: Highly efficient CO2 absorption was realized through formation of zwitterionic adducts, combining synthetic strategies to ionic liquids (ILs) and coordination. The essence of our strategy is to make use of multidentate cation coordination between Li+ and an organic base. Also PEG-functionalized organic bases were employed to enhance the CO2-philicity. The ILs were reacted with CO2 to form the zwitterionic adduct. Coordination effects between various lithium salts and neutral ligands, as well as the CO2 capacity of the chelated ILs obtained were investigated. For example, the CO2 capacity of PEG150MeBu2N increased steadily from 0.10 to 0.66 (mol CO2 absorbed per mol of base) through the formation of zwitterionic adducts being stabilized by Li+.
13. Yu-Nong Li, Liang-Nian He,* Xian-Dong Lang, Xiao-Fang Liu and Shuai Zhang, An integrated process of CO2 capture and in situ hydrogenation to formate using a tunable ethoxyl-functionalized amidine and Rh/bisphosphine system, RSC Adv., 2014, 4, 49995-50002. [link]
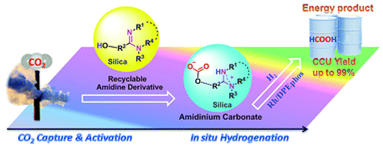
Abstract: An integrated process of CO2 capture and in situ hydrogenation into formate was achieved in 95–99% yield using a tunable ethoxyl-functionalized amidine and Rh/bisphosphine system, being regarded as an alternative carbon capture and utilization approach to supply fuel-related products, to circumvent the energy penalty in carbon capture and storage. CO2 was captured by non-volatile amidine derivatives with simultaneous activation to form zwitterionic amidinium carbonate, and subsequent hydrogenation was facilitated by Rh/bisphosphine. The adsorption capacity and hydrogenation efficiency can be optimized by tuning the ethoxyl side chain. Particularly, the alkanolamidine bearing an intramolecular hydrogen donor derived from 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) gave both a high CO2 uptake (molar ratio of 0.95:1) and excellent hydrogenation yield (99%). Furthermore, the silica-supported alkanolamidine was readily recovered and reused with the retention of good performance. This kind of carbon capture and utilization pathway could be a potential energy-saving option for industrial upgrading of CO2 from waste to fuel-related products in a carbon neutral manner.
14. Qing-Wen Song, Liang-Nian He*, Synthesis of 3-benzyl-5- methyleneoxazolidin-2-one from N-benzylprop-2-yn-1-amine and CO2 in Green Synthesis Volume 1, 2014, pp 45-53; Synthesis of the 5-membered cyclic carbonates from epoxides and CO2 in Green Synthesis Volume 1, 2014, pp 55-61 CRC Press Taylor @ Francis Group: Dallas.
15. Ran Ma, Zheng-Fen Diao, Zhen-Zhen Yang, Liang-Nian He*, Homogeneous Catalysis Promoted by Carbon Dioxide in Transformation and Utilization of Carbon Dioxide, Bhanage, Bhalchandra M.; Arai, Masahiko (Eds.); Series Editors: He, L.-N., Rogers, R.D., Su, D., Tundo, P., Zhang, Z.C., Series book Green Chemistry and Sustainable Technology(ISSN: 2196-6982), Springer: Dordrecht Heidelberg, 2014, pp 337-368. [link]
16. Yu-Nong Li, Liang-Nian He*, Zhen-Feng Diao, Zhen-Zhen Yang, Carbon Capture with Simultaneous Activation and its Subsequent Transformation, in Rudi van Eldik, Michele Aresta, editors: CO2 Chemistry, Vol 66 (ISBN 978-0-12-420221-4), ADIOCH, Burlington: Academic Press, 2014, pp. 289-345.
17. Chun-Xiang Guo, Ran Ma, Liang-Nian He*, Metal-promoted Synthesis of Cyclic Carbonates from 1,2-diols and Carbon Dioxide, The Open Organic Chemistry Journal, 2014, 8: 6-14. [link]
Abstract: Chemical fixation of CO2 to value-added products/materials/fuel has attracted more and more attention from both academia and governmental agencies all over the worldsince CO2 is an easily available and sustainable C1resource with the advantage of being abundant, nontoxic, nonflammable and renewable. Synthesis of organic carbonates starting from CO2 is one of the most promising methodologies and has been widely investigated. The use of CO2 and 1,2-diols can bring many advantages, such as reusing the byproducts generated in the industrial dimethyl carbonate (DMC) process and simplifying the post purification process. In this paper, synthetic methods with reaction mechanism of ethylene carbonate (EC), propylene carbonate (PC) and glycerol carbonate from CO2 and ethylene glycol (EG), or propylene glycol (PG) or glycerol are systematically discussed.