1. An-Hua Liu, Ran Ma, Chan Song, Zhen-Zhen Yang, Ao Yu*, Yu Cai, Liang-Nian He*, Ya-Nan Zhao, Bing Yu, Qing-Wen Song. Equimolar CO2 Capture by N-Substituted Amino Acid Salts and Subsequent Conversion, Angew. Chem. Int. Ed., 2012, 51(45), 11306-11310. [link]
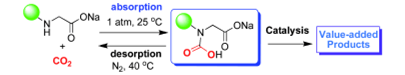
Abstract: N-substituted amino acid salts in poly(ethylene glycol) reversibly absorb CO2 in nearly 1:1 stoichiometry. Carbamic acid is thought to be the absorbed form of CO2; this was supported by NMR and in situ IR spectroscopy, and DFT calculations. The captured CO2 could be converted directly into oxazolidinones and thus CO2 desorption could be sidestepped.
2. Zhen-Zhen Yang, Liang-Nian He*, Jian Gao, An-Hua Liu and Bing Yu, Carbon dioxide utilization with C–N bond formation: carbon dioxide capture and subsequent conversion, Energy Environ. Sci., 2012, 5, 6602-6639. [link]
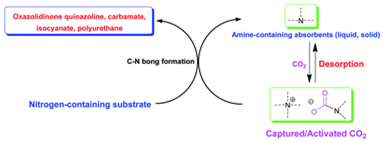
Abstract: Carbon dioxide chemistry (in particular, capture and conversion) has attracted much attention from the scientific community due to global warming associated with positive carbon accumulation. The most widely used chemical absorption technique for carbon capture and storage/sequestration (CCS) would be essentially adopting amino-containing absorbents through formation of C–N bond in terms of mechanistic consideration. However, extensive energy input in desorption and compression process would be a crucial barrier to realize practical CCS. On the other hand, CO2 is very attractive as an environmentally friendly feedstock to replace the hazardous phosgene route for making commodity chemicals, fuels, and materials from a standpoint of green chemistry, whereas the reactions involving CO2 are commonly carried out at high pressure, which may not be economically suitable and also pose safety concerns. The challenge is to develop catalysts that are capable of activating CO2 under low pressure (preferably at 1 atm), and thus incorporating CO2 into organic molecules catalytically. We have proposed a carbon capture and utilization (CCU) strategy as an alternative approach to addressing the energy penalty problem in CCS. The essence of our strategy is to use captured CO2, also considered as the activated form of CO2, which could render this system suitable for accomplishing chemical transformation of CO2 under low pressure to avoid an additional desorption step. Indeed, CO2 could be activated through the formation of carbamate/alkyl carbonate with Lewis basic nitrogen species. In this review, we would like to discuss and update advances on CCU, particularly C–N bond formation with the production of oxazolidinones, quinazolines, carbamates, isocyanates and polyurethanes by using CO2 as C1 feedstock, and CO2 capture by amino-containing absorbents, including conventional aqueous solution of amine, chilled ammonia, amino-functionalized ionic liquids and solid absorbents such as amino-functionalized silica, carbon, polymers and resin, presumably leading to CO2's activation and thus subsequent conversion through C–N bond formation pathway.
3. Jian Gao, Qing-Wen Song, Liang-Nian He*, Zhen-Zhen Yang and Xiao-You Dou, Efficient iron(III)-catalyzed three-component coupling reaction of alkyne, CH2Cl2 and amine to propargylamine, Chem. Commun., 2012. 48, 2024-2026. [link]
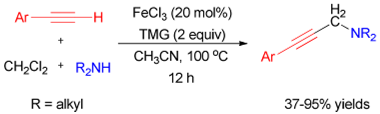
Abstract: Iron(III) was proved efficient catalyst for the three-component coupling reaction of aromatic terminal alkynes, CH2Cl2 and aliphatic secondary amines for the facile synthesis of propargylamines.
4. Bin Li, An-Hua Liu, Liang-Nian He,* Zhen-Zhen Yang, Jian Gao and Kai-Hong Chen, Iron-catalyzed selective oxidation of sulfides to sulfoxides with the polyethylene glycol/O2 system, Green Chem., 2012, 14, 130-135. [link]
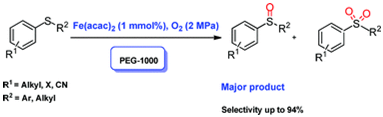
Abstract: Readily available iron compounds were found to be active catalysts for the selective oxidation of sulfide to sulfoxide with molecular oxygen as the oxidant in polyethylene glycol (PEG). As an indispensable component, PEG had a great promotive effect on the reaction. Notably, high conversion (>99%) along with excellent chemo-selectivity of up to 94% could be attained by using Fe(acac)2 as the catalyst at 100 oC. This methodology was proved to be applicable for the transformation of various aromatic and aliphatic sulfides into the corresponding sulfoxides with high selectivity. PEG is considered to play a crucial role in stablizing the Fe(IV)-oxo species formed in situ which is supposed to be responsible for the sulfide oxidation.
5. Zhen-Zhen Yang, Ya-Nan Zhao, Liang-Nian He*, Jian Gao and Zhong-Shu Yin, Highly efficient conversion of carbon dioxide catalyzed by polyethylene glycol-functionalized basic ionic liquids, Green Chem., 2012, 14, 519-527. [link]
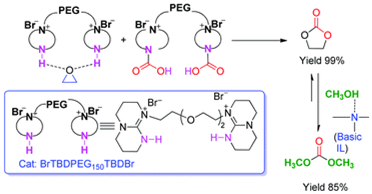
Abstract: A series of polyethylene glycol (PEG)-functionalized basic ionic liquids (ILs) were developed for efficient CO2 conversion into organic carbonates under mild conditions. In particular, BrTBDPEG150TBDBr was proven to be a highly efficient and recyclable catalyst for the synthesis of cyclic carbonates without utilization of any organic solvents or additives. This is presumably due to the activation of epoxide assisted by hydrogen bonding and activation of CO2 by the ether linkage in the PEG backbone or through the formation of carbamate species with the secondary amino group in the IL cation on the basis of in situ FT-IR study under CO2 pressure. In addition, the subsequent transesterification of cyclic carbonate e.g. ethylene carbonate (EC) with methanol to dimethyl carbonate (DMC) can also be effectively catalyzed by BrTBDPEG150TBDBr, thanks to the activation of methanol by the secondary and tertiary nitrogen in the IL to easily form CH3O−, realizing a so-called “one-pot two-stage” access to DMC from CO2without separation of cyclic carbonate by using one kind of single component catalyst. Therefore, this protocol represents a highly efficient and environmentally friendly example for catalytic conversion of CO2 into value-added chemicals such as DMC by employing PEG-functionalized basic ILs as catalysts.
6. Bing Yu, An-Hua Liu, Liang-Nian He*, Bin Li, Zhen-Feng Diao and Yu-Nong Li, Catalyst-free approach for solvent-dependent selective oxidation of organic sulfides with oxone, Green Chem., 2012, 14, 957-962. [link]
>>>Highlighted on Green Chemistry Blog
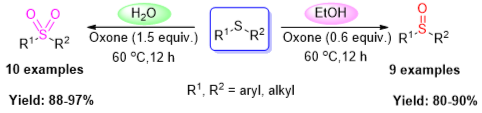
Abstract: Selective oxidation of sulfides was successfully performed by employing oxone (2KHSO5·KHSO4·K2SO4) as oxidant without utilization of any catalyst/additive under mild reaction conditions. Notably, the reaction can be controlled by the chosen solvent. When ethanol was used as the solvent, sulfoxides were obtained in excellent yield; the reaction almost exclusively gave the sulfone in water. Furthermore, this protocol worked well for various sulfides to the corresponding sulfoxides in ethanol or sulfones in water.
7. Jian Gao, Qing-Wen Song, Liang-Nian He*, Chang Liu, Zhen-Zhen Yang, Xu Han, Xue-Dong Li and Qing-Chuan Song, Preparation of polystyrene supported Lewis acidic Fe(III) ionic liquid and its application in catalytic conversion of carbon dioxide, Tetrahedron, 2012, 68, 3835-3842.[link]
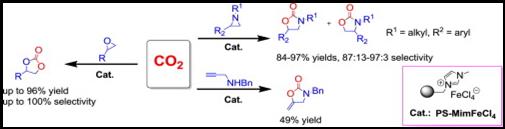
Abstract: A polystyrene supported Lewis acidic iron-containing ionic liquid was proved to be recyclable and efficient heterogeneous catalyst for converting CO2 into cyclic carbonate without utilization of any organic solvent or additive. Excellent yields and selectivity were obtained under mild reaction conditions. Notably, the catalyst could be readily recovered and reused over five times without appreciable loss of catalytic activity. A possible catalytic cycle was proposed. The present protocol has successfully been applied to reactions of aziridine/propargyl amines with CO2. This kind of the catalyst presented herein would have great potential in industrial application thanks to its featured advantages.
8. An-Hua Liu, Yu-Nong Li, and Liang-Nian He*, Organic synthesis using carbon dioxide as phosgene-free carbonyl reagent, Pure Appl. Chem. 2012, 84, 3, 581-602. (invited by editor) [link]
Abstract: CO2 is very attractive as a typical renewable feedstock for manufacturing commodity chemicals, fuel, and materials since it is an abundant, nontoxic, nonflammable, and easily available C1 resource. The development of greener chemical methodologies for replacing the utility of hazardous and environmentally undesirable phosgene largely relies on ingenious activation and incorporation of CO2 into valuable compounds, which is of paramount importance from a standpoint of green chemistry and sustainable development. Great efforts have been devoted to constructing C–C, C–O, and C–N bond on the basis of CO2 activation through molecular catalysis owing to its kinetic and thermodynamic stability. The aim of this article is to demonstrate the versatile use of CO2 in organic synthesis as the alternative carbonyl source of phosgene, with the main focus on utilization of CO2 as phosgene replacement for the synthesis of value-added compounds such as cyclic carbonates, oxa-zolidinones, ureas, isocyanates, and polymers, affording greener pathways for future chemical processes.
9. An-Hua Liu, Ran Ma, Meng Zhang, Liang-Nian He*, In situ acidic carbon dioxide/water system for selective oxybromination of electron-rich aromatics catalyzed by copper bromide. Catal. Today, 2012, 194(1), 38-43.[link]
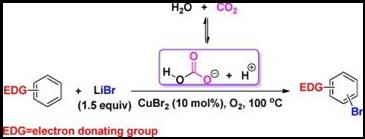
Abstract: Carbon dioxide, being one of the major greenhouse gases responsible for global warming, its atmospheric level grows ever faster since the beginning of industrial era. Great efforts have been devoted to developing versatile technologies/processes to adjust and manipulate the rapid growth of CO2 emission. Besides CO2 capture and storage/sequestration (CCS) to control its emission, chemical utilization of the captured CO2 (CCU) emerges to be a rational technique for economical benefits as well as environmental concerns. As for the aim of CO2 utilization, an environmentally benign CO2/water reversible acidic system was developed for the copper (II)-catalyzed selective oxybromination of electron-rich aromatics without the need of any conventional acid additive and organic solvent. Notably, up to 95% yields of the bromination products were attained with good regio-selectivity when cupric bromide was used as the catalyst and lithium bromide as a cheap and easy handling bromine source under supercritical CO2. The catalytic system worked well for electron-rich aromatics including ethers, sulfides and mesitylene. Carbonic acid in situ formed from CO2 and water is supposed to act as the proton donator in the Brønsted acid-promoted reaction. Notably, CO2 in this study serves as a reaction medium and a promoter in conjunction with water and also provides safe environment for aerobic reactions. Given with excellent reaction efficiency as well as no need of neutralization disposal, this process thus represents an environmentally friendly approach for aerobic bromination of aromatics with essential reduction of CO2 emission as well as an economically beneficial way for application of captured CO2.
10. Xiao-Yong Dou, Liang-Nian He*, Zhen-Zhen Yang, Proline-Catalyzed Synthesis of 5-Aryl-2-Oxazolidinones from Carbon Dioxide and Aziridines under Solvent-Free Conditions, Synth. Commun., 2012, 42, 62-74. [link]
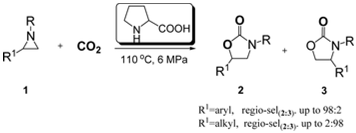
Abstract: Natural α-amino acids were proven to be ecofriendly and recyclable catalysts for the carboxylation of aziridines with CO2 without utilization of any organic solvents or additives. Notably, a series of 5-aryl-2-oxazolidinones were obtained in good yield together with excellent chemo- and regioselectivity under mild conditions using proline as the catalyst. Notably, the catalyst could be recycled more than five times after a simple separation procedure without appreciable loss of catalytic activity. This process represents a promising strategy for homogeneous catalyst recycling.
11. Yu-Nong Li, Jin-Quan Wang*, Liang-Nian He*, Zhen-Zhen Yang, An-Hua Liu, Bing Yu and Chao-Ran Luan. Experimental and theoretical studies on imidazolium ionic liquid-promoted conversion of fructose to 5-hydroxymethylfurfural, Green Chem., 2012, 14, 2752-2758. [link]

Abstract: A combined experimental and computational study on the imidazolium ionic liquid-promoted conversion of fructose to 5-hydroxymethylfurfural (HMF) was performed. In particular, 1-butyl-3-methyl-imidazolium bromide (BMImBr) was found to be unexpectedly effective for conversion of fructose into HMF without utilizing any other additive or catalyst. Under the optimized conditions, nearly 100% conversion of fructose with a 95% yield of HMF could be obtained. In addition, BMImBr could be easily recovered and reused over 6 times without significant loss of activity. This protocol represents a simple, recyclable and environmentally friendly pathway for HMF production. Furthermore, the detailed mechanism of the BMcImBr-promoted conversion of fructose into HMF was also studied through an in situ FT-IR technique, NMR and density functional theory calculations, and demonstrated that the hydrogen bond interaction between BMImBr and fructose could play an important role in promoting the dehydration of fructose. This work also provides further understanding at the molecular level of the reaction process for ionic liquid-promoted conversion of fructose to HMF.
12. Zhen-Zhen Yang, Liang-Nian He*, Qing-Wen Song, Kai-Hong Chen, An-Hua Liu and Xiang-Ming Liu, Highly efficient SO2 absorption/activation and subsequent utilization by polyethylene glycol-functionalized Lewis basic ionic liquids, Phys. Chem. Chem. Phys., 2012, 14, 15832-15839. [link]
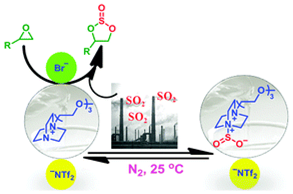
Abstract: Up to now, flue-gas desulfurization (FGD) is one of the most effective techniques to control SO2 emission from the combustion of fossil fuels. The conventional technology for FGD poses serious inherent drawbacks such as formation of byproducts and volatilization of solvents. In this work, polyethylene glycol (PEG)-functionalized Lewis basic ionic liquids (ILs) derived from DABCO were proved to be highly efficient absorbents for FGD due to its specific features such as high thermal stability, negligible vapor pressure, high loading capacity. Notably, PEG150MeDABCONTf2 gave an extremely high SO2 capacity (4.38 mol mol−1 IL), even under 0.1 bar SO2 partial pressure (1.01 mol mol−1 IL), presumably owing to the strong SO2-philic characterization of the PEG chain. Furthermore, the absorbed SO2 could be easy to release by just bubbling N2 at room temperature, greatly reducing energy requirement for SO2 desorption. In addition, SO2/CO2 selectivity (110) of PEG150MeDABCONTf2 is two times larger than the non-functionalized imidazolium IL (45). On the other hand, through activation of SO2 with the tertiary nitrogen in the cation, Lewis basic ILs such as PEG150MeDABCOBr proved to be efficient catalysts for the conversion of SO2 to some value-added chemicals such as cyclic sulfites without utilization of any organic solvent or additive. Thus, this protocol would pave the way for the development of technological innovation towards efficient and low energy demanded practical process for SO2 absorption and subsequent transformation.
13. Zhen-Zhen Yang, Liang-Nian He*, An-Hua Liu and Yu-Nong Li, Catalytic fixation of carbon dioxide into fuel and chemicals, in Kirk-Othmer Encyclopedia: Carbon Dioxide, Chemical Fixation, John Wiley & Sons: NJ, 2012. [link]
14. Zhen-Zhen Yang, Qing-Wen Song, Liang-Nian He*, Capture and Utilization of Carbon Dioxide with Polyethylene Glycol, (SpringerBriefs in Molecular Science/SpringerBriefs in Green Chemistry for Sustainability), ISBN-10: 3642312675. [link]
15. Qing-Wen Song, Ya-Nan Zhao, Liang-Nian He*, Jian Gao, Zhen-Zhen Yang, Synthesis of Oxazolidinones/Polyurethanes from Aziridines and CO2, Current Catalysis, 2012, 1(2), 107-124. [link]
Abstract: From the viewpoint of sustainable development, chemical fixation/utilization of CO2 in an environmentally benign manner has been drawing much interest, especially in the field of organic chemistry. In this review, 100% atom economical routes such as the reaction of CO2 and aziridines to prepare oxazolidinones and polyurethanes with more consideration given to the catalytic strategies and insight into the reaction mechanism are reviewed in the context of CO2 conversion into useful chemicals. High atom efficiency of the reaction and wide application of the CO2-derived products are the main driving force for the constant interest paid to catalytic conversion of CO2 into fuels, value-added chemical, polycarbonate materials.
16. Zhen-Zhen Yang, Ya-Nan Zhao, Liang-Nian He*, Task-specific ionic liquid-catalyzed conversion of carbon dioxide into fuel additive and value-added chemicals, in Handbook of Ionic Liquid: Properties, Applications and Hazards (ISBN 978-1-62100-349-6); Mun, J., and Sim, H., Ed.; Nova Science Publishers: NY, 2012, p. 227-256. [link]