1. Bing Yu and Liang-Nian He*. Upgrading Carbon Dioxide by Incorporation into Heterocycles, ChemSusChem, 2015, 8(1): 52-62. [link]
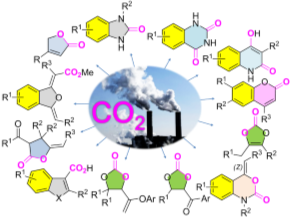
Abstract: CO2 in heterocycles: Direct incorporation of CO2 as the entire “CO2” moiety or “C=O” fragments into organic substrates can be successfully performed by carbonylative/carboxylative cyclization of the nitrogen/oxygen/carbon-nucleophilic species with CO2 or initiated by C-H bond activation, resulting in the formation of various heterocycles. These techniques stimulate further interest in upgrading CO2 from a waste into an ideal and sustainable chemical feedstock in organic synthesis.
2. Qing-Wen Song, Wei-Qiang Chen, Ran Ma, Ao Yu, Qiu-Yue Li, Yao Chang and Liang-Nian He*, Bifunctional Silver(I) Complex-Catalyzed CO2 Conversion at Ambient Conditions: Synthesis of α-Methylene Cyclic Carbonates and Derivatives, ChemSusChem, 2015, 8(5): 821-827. [link]
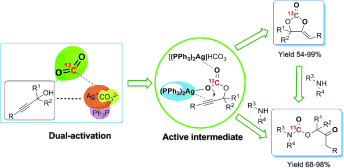
Abstract: The chemical conversion of CO2 at atmospheric pressure and room temperature remains a great challenge. The triphenylphosphine complex of silver(I) carbonate was proved to be a robust bifunctional catalyst for the carboxylative cyclization of propargylic alcohols and CO2 at ambient conditions leading to the formation of α-methylene cyclic carbonates in excellent yields. The unprecedented performance of [(PPh3)2Ag]2CO3 is presumably attributed to the simultaneous activation of CO2 and propargylic alcohol. Moreover, the highly compatible basicity of the catalytic species allows propargylic alcohol to react with CO2 leading to key silver alkylcarbonate intermediates: the bulkier [(Ph3P)2AgI]+ effectively activates the carbon–carbon triple bond and enhances O-nucleophilicity of the alkylcarbonic anion, thereby greatly promoting the intramolecular nucleophilic cyclization. Notably, this catalytic protocol also worked well for the reaction of propargylic alcohols, secondary amines, and CO2 (at atmospheric pressure) to afford β-oxopropylcarbamates.

3. Chun-Xiang Guo, Bing Yu, Jia-Ning Xie and Liang-Nian He*, Silver tungstate: A single-component bifunctional catalyst for carboxylation of terminal alkynes with CO2 in ambient conditions. Green Chem., 2015, 17(1): 474-479. [link]
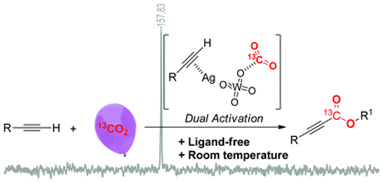
Abstract: Silver tungstate was successfully developed as a bifunctional catalyst for the ligand-free carboxylation of various terminal alkynes with electron-withdrawing or electron-donating groups under atmospheric pressure of carbon dioxide (CO2) at room temperature. In this protocol, dual activation – i.e., the terminal alkyne activated by silver, and CO2 activation by the tungstate anion – was verified using nuclear magnetic resonance spectroscopy, and means that this reaction can be run under ambient conditions. Notably, this protocol can be applied to the preparation of a phenylacrylate derivative by a cascade reaction using phenylacetylene, CO2 and benzylamine as starting materials.
4. An-Hua Liu, Bing Yu and Liang-Nian He*. Catalytic Conversion of Carbon Dioxide to Carboxylic Acid Derivatives. Greenhouse Gas Sci Technol. 2015, 5, 17-33. [link]
Abstract: Chemical utilization of the greenhouse gas, i.e., CO2 as an abundant, easily available, and renewable carbon feedstock for producing chemicals, materials, and fuels is attractive as an integral part of the carbon cycle. Based on the principle of CO2 activation and elaborately designed catalysts, various transformation of CO2 can be realized under mild conditions. In particular, establishing practical methodologies for catalytic carboxylation by CO2 as carboxylative reagent would be a fascinating dream for synthetic chemists. This review covers the updated advances in the catalytic conversion of CO2 into carboxylic acid derivatives from alkenes, alkynes, aromatic halides, aromatic C(sp2)-H bonds, etc.
5. Xian-Dong Lang, Shuai Zhang, Qing-Wen Song and Liang-Nian He*, Tetra-butylphosphonium arginine-based ionic liquid-promoted cyclization of 2-aminobenzonitrile with carbon dioxide, RSC Adv., 2015, 5, 15668-15673. [link]
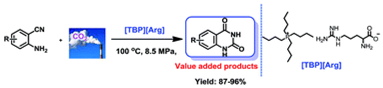
Abstract: An easily prepared amino acid ionic liquid (AAIL) i.e. [TBP][Arg] comprising a tetra-butylphosphonium cation and an arginine anion was found to be an efficient and recyclable catalyst for the synthesis of quinazoline-2,4(1H,3H)-diones from 2-aminobenzonitriles and CO2 under solvent-free conditions. As a result, various 2-aminobenzonitriles bearing electron-withdrawing or electron-donating substituents worked well to afford quinazoline-2,4(1H,3H)-diones in excellent yields. Notably, this type of AAIL showed good stability, and could be easily recovered and reused five times without significant loss of its catalytic activity. This process represents an alternative approach for greener chemical fixation of CO2 to afford valuable compounds.
6. Xiu-Zhen Lin, Zhen-Zhen Yang, Liang-Nian He and Zhong-Yong Yuan*, Mesoporous zirconium phosphonates as efficient catalysts for chemical CO2 fixation. Green Chem., 2015, 17, 795-798. [link]
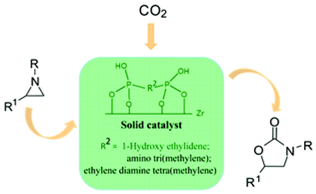
Abstract: Mesoporous zirconium phosphonates were demonstrated as highly effective catalysts for the heterogeneously catalyzed cycloaddition reaction between aziridines and CO2 to yield oxazolidinones in a solvent-free system without introducing any co-catalysts or halogen species, exhibiting outstanding activity and selectivity, as well as excellent recyclability.
7. Siyang Liu, Qingqing Zhu, Qingxin Guan, Liangnian He, Wei Li*, Bio-aviation fuel production from hydroprocessing castor oil promoted by the nickel-based bifunctional catalysts, Bioresource Technology, 2015 183: 93-100. [link]
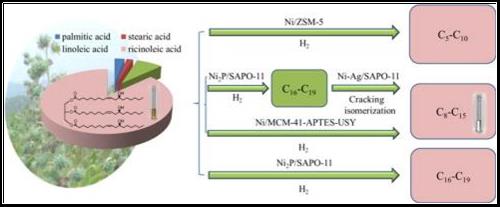
Abstract: Bio-aviation fuel was firstly synthesized by hydroprocessing castor oil in a continuous-flow fixed-bed microreactor with the main objective to obtain the high yield of aviation fuel and determine the elemental compositions of the product phases as well as the reaction mechanism. Highest aviation range alkane yields (91.6 wt%) were achieved with high isomer/n-alkane ratio (i/n) 4.4–7.2 over Ni supported on acidic zeolites. In addition, different fuel range alkanes can be obtained by adjusting the degree of hydrodeoxygenation (HDO) and hydrocracking. And the observations are rationalized by a set of reaction pathways for the various product phases.
8. Chun-Xiang Guo, Bing Yu, Ran Ma and Liang-Nian He*, Metal-promoted Carboxylation of Alkynes/allenes with Carbon Dioxide. Current Green Chemistry, 2015, 2(1), 14-25. [link]
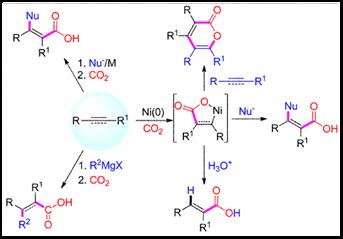
Abstract: Carbon dioxide is generally known as one of the main greenhouse gasses which have caused a series of environmental problems. On the other hand, CO2 can also be regard as an abundant, nontoxic, renewable and economical C1-synthon in organic synthesis. Chemical utilization of CO2 has attracted more and more attentions all over the world and has been studied extensively and intensely for decades. Meanwhile, acrylic acid is a valuable chemical which has been widely used in industry. From the point of sustainable development, the direct installation of CO2 into organic compounds to afford carboxylic acid derivatives is one of the most concise and promising ways for CO2 utilization. In this article, advances in the metal-promoted carboxylation reaction of alkynes and allenes with carbon dioxide are highlighted with mechanistic understanding on a molecular level.
9. Xian-Dong Lang, Xiao-Fang Liu and Liang-Nian He*, Sustainable Solid Catalysts for Cyclic Carbonate Synthesis from CO2 and Epoxide, Current Organic Chemistry, 2015, 19, 681-694. [link]
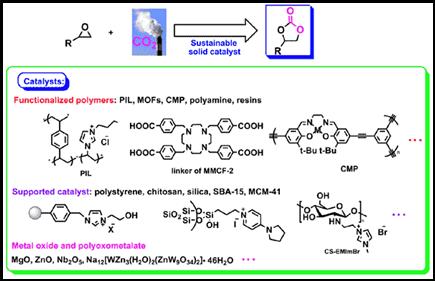
Abstract: As a major contributor to greenhouse effect, detrimental influence of CO2 has received more and more attention. Meanwhile, CO2 is also a valuable C1 building block, with several distinguished features such as low cost, nontoxicity, nonflammability, renewal, abundance. CO2 fixation is one of the most important priorities of the scientific community dedicated to reduce global warming. The last few decades have witnessed enormous attention utilizing CO2 to produce a variety of organic molecules, such as cyclic carbonates, which hold diverse applications as polar aprotic solvents, intermediates for organic and polymeric synthesis, and chemical ingredients for pharmaceutical/ fine chemicals in biomedical applications, electrolytic elements of lithium secondary batteries. Development of sustainable solid catalysts for the cycloaddition of epoxide with CO2 into cyclic carbonates is of paramount importance from a viewpoint of C1 chemistry and green chemistry. In this mini-review, we would like to update the recent advances in development of efficient heterogeneous catalysts including polymeric ionic liquids, metal organic frameworks, conjugated microporous polymer, polyamines, functionalized ion-exchange resins, polystyrene-supported ionic liquids, chitosan-based heterogeneous catalysts, silica-supported catalysts, magnetic nanoparticle supported catalysts, metal oxide and polyoxometalate, and others for the cycloaddition of epoxide with CO2, and we hope this presentation will stimulate further interest in chemical conversion of CO2 using sustainable catalysts.
10. 李雨浓, 何良年*. 二氧化碳的原位催化氢化反应. 科学通报, 2015, 60(16): 1465-1487. [link]
摘要:二氧化碳(CO2)的吸收和封存技术是规模化减缓CO2排放的手段之一, 但其脱附、压缩、运输和储存过程中, 不可避免地消耗能量. 同时, CO2作为无毒无害、廉价易得的C1资源, 可代替传统羰基化试剂合成高附加值的化工产品. 因此, CO2变废为宝, 高值化利用的研究, 特别是将CO2还原为甲酸、甲醇等能源类产品, 具有重要科学意义及应用价值. 着眼于CO2吸收和资源化利用相结合的策略, 将CO2的吸收产物进行原位催化反应, 既可绕过脱附、压缩环节; 又可消除高压反应的不足、减少设备投入及节能降耗; 同时, 吸收过程中CO2分子得到活化, 有利于后续化学转化反应在低压温和条件下进行. 催化氢化反应在多种CO2资源化利用途径中具有重要意义和应用前景, 将CO2的吸收产物进行原位催化氢化反应能够成功获得甲酸、甲醇等重要的能源产品. 本文概括介绍了CO2的捕集方法及其化学转化为衍生物的路径, 总结了CO2氢化反应的催化体系和作用机制, 在此基础上, 重点分析讨论了CO2的原位催化氢化反应机理和最新进展.
11. Bing Yu, Jia-Ning Xie, Chun-Lai Zhong, Wei Li,* and Liang-Nian He*, Copper(I)@Carbon-Catalyzed Carboxylation of Terminal Alkynes with CO2 at Atmospheric Pressure. ACS Catal. 2015, 5, 3940-3944. [link]

Abstract: Activated carbon supported CuBr was found to be an efficient catalyst for the carboxylation of terminal alkynes under atmospheric pressure of CO2 using ethylene carbonate as solvent at 80 °C for only 2 h, as verified with 13CO2. Various terminal alkynes could react smoothly with CO2 and organic halides under the reaction conditions to afford the corresponding carboxylic esters. In addition, the catalyst can be easily recovered and reused at least five times without significant loss of activity.
12. Jia-Ning Xie, Bing Yu, Chun-Xiang Guo and Liang-Nian He*, Copper(I)/phosphine-catalyzed tandem carboxylation/annulation of terminal alkynes with ambient pressure of CO2: one-pot access to 3a-hydroxyisoxazolo[3,2-a]isoindol-8(3aH)-ones. Green Chem., 2015, 17(7), 4061-4067. [link]
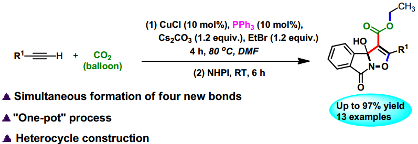
Abstract: An efficient method for the synthesis of 3a-hydroxyisoxazolo[3,2-a]isoindol-8(3aH)-ones from CO2, terminal alkynes, EtBr, and NHPI (N-hydroxyphthalimide) was developed through the tandem carboxylation/annulation strategy catalyzed by copper(I)/phosphine system. This one-pot multicomponent reaction conducted at atmospheric CO2 pressure to afford the target products in good to excellent yields under mild conditions. Notably, a wide range of functional groups were tolerated in this procedure. This protocol with simultaneous formation of four novel bonds i.e. two C-C band and two C-O bond represents an efficient methodology for upgrading CO2 into heterocycles.
13. Shuai Zhang, Wei-Qiang Chen, Ao Yu* and Liang-Nian He*, Palladium-Catalyzed Carboxylation of Benzyl Chlorides with Atmospheric Carbon Dioxide in Combination with Manganese/Magnesium Chloride, ChemCatChem, 2015, 7(23), 3972-3977. [link]
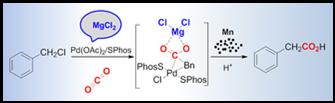
Abstract: An efficient direct carboxylation of a series of benzyl chlorides with CO2 catalyzed by Pd(OAc)2/dicyclohexyl (2′,6′-dimethoxy-[1,1′-biphenyl]-2-yl)phosphine (SPhos) was developed to afford the corresponding phenylacetic acids in combination with Mn powder as a reducing reagent and MgCl2 as an indispensable additive. The reaction proceeded smoothly under 1atm CO2. The application of Mn powder instead of a sensitive reducing reagent represents an operationally simple access to phenylacetic acids. Notably, MgCl2 is able to stabilize the (SPhos)2PdII(Bn)(Cl)(η1-CO2)(MgCl2) adduct and thus facilitates CO2 insertion into the PdII-C bond, which is supported by a DFT study.
14. Qing-Wen Song, Liang-Nian He*, Transition Metal-Promoted CO2 Conversion under Mild Reaction Conditions in Advances in CO2 Capture, Sequestration, and Conversion, Vol. 1194, American Chemical Society, 2015, pp. 47-70. [link]
Abstract: Carbon dioxide can be regarded as an inexpensive, abundant sustainable feedstock for producing value-added chemicals. Therefore, development of efficacious processes using CO2 as chemical feedstock under mild conditions particularly low CO2 pressure (ideally at 1 bar) could be still highly desirable. Accordingly, only if we understand the underlying principles of CO2 activation can the goal of using CO2 as an environmentally friendly and economically feasible source of carbon under mild reaction conditions be achieved. In this regard, transition metal catalysis based on CO2 activation for efficient chemical transformation of CO2 with outstanding selectivities is appealing from a standpoint of sustainable chemistry. In this context, great efforts have been devoted to constructing C-N and C-O bond on the basis of selecting high-energy starting materials and CO2 activation through molecular catalysis to overcome its thermodynamic stability and kinetic inertness. The aim of this chapter is to draw attention to the chemical fixation of CO2 with propargylic alcohols or propargylmines to a-alkylene cyclic carbonates, β-oxoalkylcarbamates and 2-oxazolidinones through various transition metal catalysis. This is an attractive strategy to utilize CO2 to produce useful products. A thorough overview of the catalytic utilization of CO2 with propargylic alcohols with gaining insights into the reaction mechanism is also presented.
15. Qing-Wen Song, Zhi-Hua Zhou, Hong Yin, Liang-Nian He*, Silver(I)-Catalyzed Synthesis of β-Oxopropylcarbamates from Propargylic Alcohols and CO2 Surrogate: A Gas-Free Process, ChemSusChem, 2015, 8(23), 3967-3972. [link]
Abstract: The utilization of carbon dioxide poses major challenges owing to its high thermodynamic stability and kinetic inertness. To circumvent these problems, a simple reaction system is reported comprising ammonium carbamates as carbon dioxide surrogates, propargylic alcohols, and a silver(I) catalyst, for the effective conversion of a wide range of alcohols and secondary amines into the corresponding β-oxopropylcarbamates. A key feature of this strategy includes quantitative use of a carbon resource with high product yields under gas-free and mild reaction conditions. Notably, this catalytic protocol also works well for the carboxylative cyclization of propargylic amines and carbon dioxide surrogates to afford 2-oxazolidinones.
16. Jia-Ning Xie, Bing Yu, Zhi-Hua Zhou, Hong-Chen Fu, Ning Wang, Liang-Nian He*, Copper(I)-based ionic liquid-catalyzed carboxylation of terminal alkynes with CO2 at atmospheric pressure, Tetrahedron Lett. 2015, 56(50), 7059-7062. [link]
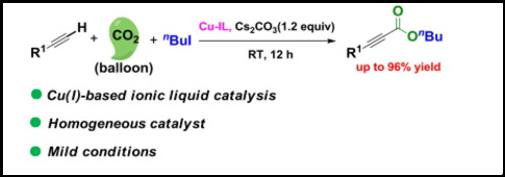
Abstract: An ionic liquid containing copper(I) proved to be an effective homogeneous catalyst for the carboxylation of terminal alkynes with ambient CO2. This developed procedure needs no external ligands and terminal alkynes with various groups proceeded smoothly at atmospheric CO2 pressure and room temperature. Interestingly, the ILs containing copper(I) in both the anion and the cation showed much higher activity in comparison with the counterparts incorporating copper(I) solely in the form of halocuprate, that is, copper(I) in the anion. Especially, activated effect of the terminal alkyne by the ionic liquid was also validated by the NMR technique.