1. 何良年,二氧化碳化学,北京:科学出版社,2013年第一版.前言
2. Qing-Wen Song, Liang-Nian He*, Jin-Quan Wang, Hiroyuki Yasuda and Toshiyasu Sakakura*, Catalytic fixation of CO2 to cyclic carbonates by phosphonium chlorides immobilized on fluorous polymer, Green Chem., 2013, 15, 110-115. [link]
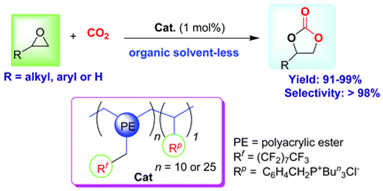
Abstract: Phosphonium chloride covalently bound to the fluorous polymer is proved to be an efficient and recyclable homogeneous CO2-soluble catalyst for organic solvent-free synthesis of cyclic carbonates from epoxides and CO2 under supercritical CO2 conditions. The catalyst can be easily recovered by simple filtration after reaction and reused with retention of high activity and selectivity. In addition, the effects of various reaction variables on the catalytic performance are also discussed in detail. The process represents a simpler access to preparing cyclic carbonates with the ease of homogeneous catalyst recycling.
3. Ya-Nan Zhao, Zhen-Zhen Yang, Si-Hang Luo and Liang-Nian He*, Design of task-specific ionic liquids for catalytic conversion of CO2 with aziridines under mild conditions, Catal. Today, 2013, 200, 2-8. [link]
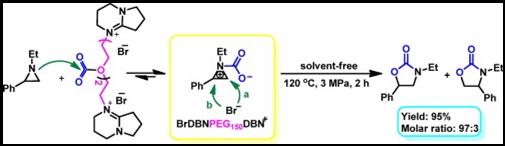
Abstract: A series of polyethylene glycol (PEG)-functionalized ionic liquids (ILs) were developed as recyclable and efficient catalysts for selective synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2 without addition of any organic solvents or additives. In particular, high yields, chemo- and regio-selectivities of oxazolidinones were attained when BrDBNPEG150DBNBr (DBN: 1,5-diazabicyclo[4.3.0]non-5-ene) was used as the catalyst, presumably due to activation of CO2 by the ether linkage in the PEG backbone, and stabilization of the ring-opened species of aziridine by the delocalized cation BrDBNPEG150DBN+. Furthermore, the catalyst could be reused for over four consecutive cycles without appreciable loss of catalytic activity and selectivity. The effects of catalyst structure and various reaction parameters on the catalytic performance were also investigated in detail. It was demonstrated that the catalyst worked well for a variety of aziridines producing the corresponding oxazolidinones in good yields and excellent regio-selectivities. Therefore, this solvent-free process could thus represent an environmentally friendly approach for ILs-catalyzed conversion of CO2 into value-added chemicals.
4. Zhen-Zhen Yang, Liang-Nian He*, Ya-Nan Zhao and Bing Yu, Highly Efficient SO2 Absorption and Its Subsequent Utilization by Weak Base/Polyethylene Glycol Binary System, Environ. Sci. Technol., 2013, 47(3), 1598-1605. [link]
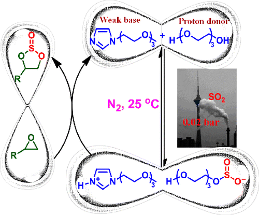
Abstract: A binary system consisting of polyethylene glycol (PEG, proton donor)/PEG-functionalized base with suitable basicity was developed for efficient gas desulfurization (GDS) and can be regarded as an alternative approach to circumvent the energy penalty problem in the GDS process. High capacity for SO2 capture up to 4.88 mol of SO2/mol of base was achieved even under low partial pressure of SO2. Furthermore, SO2 desorption runs smoothly under mild conditions (N2, 25 °C) and no significant drop in SO2 absorption was observed after five-successive absorption–desorption cycles. On the other hand, the absorbed SO2 by PEG150MeIm/PEG150, being considered as the activated form of SO2, can be directly transformed into value-added chemicals under mild conditions, thus eliminating the energy penalty for SO2 desorption and simultaneously realizing recycle of the absorbents. Thus, this SO2 capture and utilization (SCU) process offers an alternative way for GDS and potentially enables the SO2 conversion from flue gas to useful chemicals as a value-added process.
5. Ran Ma, An-Hua Liu, Cheng-Bin Huang, Xue-Dong Li and Liang-Nian He*, Reduction of sulfoxides and pyridine-N-oxides over iron powder with water as hydrogen source promoted by carbon dioxide, Green Chem., 2013, 15(5), 1274-1279. [link]

Abstract: A green process was developed for efficient reduction of sulfoxides and pyridine-N-oxides using the iron powder in the presence of H2O/CO2 to sulfides and pyridines, respectively. Notably, H2O is employed as terminal hydrogen source, and CO2 could enhance hydrogen generation through in situ formation of carbonic acid. Thus carbonic acid offers simple neutralization by depressurizing CO2 and the system can eliminate unwanted byproducts. The high generality and chemo-selectivity of this protocol were demonstrated by the scope of substrates, in which chlorine, vinyl group and benzene ring can be tolerated.
6. Bing Yu, Zhen-Feng Diao, Chun-Xiang Guo, Liang-Nian He*, Carboxylation of olefins/alkynes with CO2 to industrially relevant acrylic acid derivatives, J. CO2 Utilization, 2013, 1, 60-68. (invited by editor) [link]
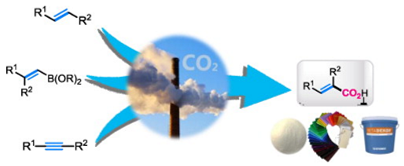
Abstract: Carbon dioxide utilization has continued to capture the interest of chemists worldwide due to global warming associated with positive carbon accumulation. As an environmentally friendly C1 feedstock, the reaction of carbon dioxide has been extensively investigated for several decades. On the other hand, acrylic acid is a valuable industrial product that is widely used for various important purposes in industry. From the point view of atom- and process-economical chemistry, the most concise and promising route for acrylic acid derivatives synthesis would be direct carboxylation of olefins or alkynes with carbon dioxide. In this review, we would like to discuss and update the latest advances on synthesis of acrylic acid derivatives from unsaturated hydrocarbons and carbon dioxide.
7. Zhenfeng Diao, Bin Li, Bing Yu, Anhua Liu, Liangnian He*, Polyethylene glycol radical-initiated aerobic propargylic oxidation in dense carbon dioxide, J. Energy Chem., 2013, 22(3): 363-367. [link]
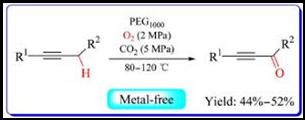
Abstract: The selective aerobic oxidation of alkynes to corresponding α,β-acetylenic ketones was achieved in polyethylene glycol/dense CO2/O2 biphasic system without any catalyst or additive. The effects of reaction parameters, e.g. temperature, CO2 pressure, PEG molecular weight and loading on the reaction were carefully examined. Moreover, various substrates worked well in the presence of PEG1000 under 5 MPa of CO2 and 2 MPa of O2 at 100 oC for 12 to 24 h and acceptable yield and selectivity could be obtained in most cases. Preliminary mechanistic investigations were also discussed.

8. Bing Yu, Zhen-Feng Diao, Chun-Xiang Guo, Chun-Lai Zhong, Liang-Nian He*, Ya-Nan Zhao, Qing-Wen Song, An-Hua Liu and Jin-Quan Wang*. Carboxylation of terminal alkynes at ambient CO2 pressure in ethylene carbonate. Green Chem., 2013, 15(9), 2401-2407. [link]

Abstract: The CuI-catalyzed carboxylation of terminal alkynes with CO2 and alkyl halides using ethylene carbonate as solvent under mild conditions was studied. DFT calculations reveal that the energy barrier of CO2 insertion into sp-hybridized Cu-C bond could be reduced by employing ethylene carbonate as solvent. Notably, the procedure was conducted under ambient CO2 pressure without external ligands. A broad range of substrates with electron-withdrawing group or electron-donating group gave the corresponding products in reasonable yields.
9. Qing-Wen Song, Bing Yu, An-Hua Liu, Ying He, Zhen-Zhen Yang, Zhen-Feng Diao, Qing-Chuan Song, Xue-Dong Li and Liang-Nian He*. PEG400-enhanced synthesis of gem-dichloroaziridines and gem-dichlorocyclopropanes through the dichlorocarbene in situ generated. RSC Adv., 2013, 3, 19009-19014. [link]
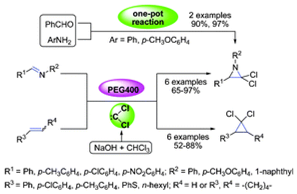
Abstract: PEG400 is employed as an efficient phase transfer catalyst for the cycloaddition reaction of imines with dichlorocarbene, which is generated in situ from chloroform and sodium hydroxide, to give gem-dichloroaziridines in moderate to excellent yields at ambient temperature. This protocol is also extended to the synthesis of cyclopropanes from a variety of alkenes. In this study, PEG400 behaves as a phase transfer reagent thanks to its ability to coordinate with alkali metal cations. Notably, the one-pot synthesis of gem-dichloroaziridines from benzaldehyde and aromatic amines has also been successfully performed. The in situ generated acid, derived from CO2 and H2O, can also be effectively applied to promote the amide synthesis via the gem-dichloroaziridine pathway. The application of the gem-dichlorocyclopropane as a platform chemical is also briefly demonstrated, to afford the 2-phenylacrylaldehyde derivative via a ring-opening reaction.
10. Yu-Nong Li, Liang-Nian He*, An-Hua Liu, Xian-Dong Lang, Zhen-Zhen Yang, Bing Yu and Chao-Ran Luan. In situ hydrogenation of the captured CO2 to formate with polyethyleneimine and Rh/monophosphine system. Green Chem., 2013, 15, 2825-2829. [link]
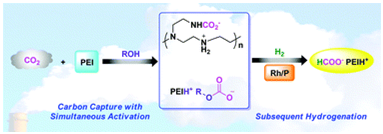
Abstract: CO2 in the air can be efficiently captured with simultaneous activation by PEI (polyethyleneimine) to form ammonium carbamate and/or carbonate species. Thus, the in situ hydrogenation of captured CO2 into energy-storage materials rather than going through the desorption of conventional CCS (carbon capture and storage) runs better in comparison with equivalent gaseous CO2, thus validating this potential application of CCU (carbon capture and utilization) for supplying renewable energy. PEI600 as an effective carbon absorbent in this study could also be assumed to serve as both ligand and base to promote the catalytic hydrogenation of captured CO2, consequently acting as a ‘hinge base’ to combine capture and hydrogenation processes. The pathway was studied by NMR and in situ FT-IR spectroscopy under CO2 pressure. This protocol could open up great potential in transforming the captured CO2 from waste to fuel-related products.
11. An-Hua Liu, Jian Gao, Liang-Nian He*, Catalytic Activation and Conversion of Carbon Dioxide into Fuels/ Value-Added Chemicals Through C-C Bond Formation, in New and Future Developments in Catalysis. Activation of Carbon Dioxide, 1st edition. Suib, S. Ed.; Elsevier Inc.: Philadelphia, 2013, p81-147. [link]