1. Cheng-Xia Miao, Liang-Nian He*, Jing-Lun Wang, Fang Wu, Self-Neutralizing in Situ Acidic CO2/H2O System for Aerobic Oxidation of Alcohols Catalyzed by TEMPO Functionalized Imidazolium Salt/NaNO2, J. Org. Chem., 2010, 75(1), 257-260. [link]
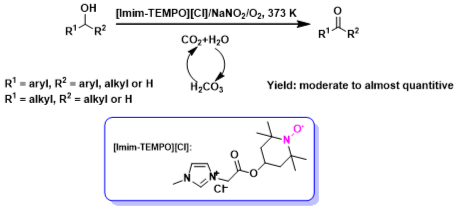
Abstract: A recyclable TEMPO functionalized imidazolium salt ([Imim-TEMPO][Cl]) and NaNO2 as the NO resource to activate dioxygen were developed to selectively oxidize a series of aliphatic, allylic, heterocyclic and benzylic alcohols to the respective carbonyl compounds in a reversible in situ acidic CO2/H2O system, which avoids using any acid, whereby can eliminate unwanted byproducts, facilitate reaction and ease separation of the catalyst and product.
2. Zhen-Zhen Yang, Liang-Nian He,* Cheng-Xia Miao, and Sébastien Chanfreau, Lewis Basic Ionic Liquids-Catalyzed Conversion of Carbon Dioxide to Cyclic Carbonate, Adv. Synth. Catal., 2010, 352, 2233-2240. [link]
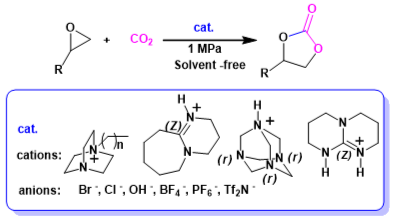
Abstract: A series of easily prepared Lewis basic ionic liquids were developed for cyclic carbonate synthesis from epoxide and carbon dioxide at low pressure without utilization of any organic solvents or additives. Notably, quantitative yield together with excellent selectivity were attained while 1,8-diazabicyclo[5.4.0]undec-7-enium chloride ([HDBU]Cl) was used as a catalyst. Furthermore, the catalyst could be recycled over five times without appreciable loss of catalytic activity. The effects of the catalyst structure and various reaction parameters on the catalytic performance were in detail investigated. This protocol was found to be applicable to a variety of epoxides producing the corresponding cyclic carbonates in high yields and selectivity. Therefore, this solvent-free process thus represents an environmentally friendly example for catalytic conversion of carbon dioxide into value-added chemicals by employing Lewis basic ionic liquids as catalyst. A possible catalytic cycle for hydrogen bond-assisted ring-opening of epoxide and carbon dioxide’s activation induced by nucleophilic tertiary nitrogen of the ionic liquid was also proposed.
3. Xiao-Yong Dou, Qi Shuai, Liang-Nian He,* Chao-Jun Li,* Copper(II) Triflate Catalyzed Three-Component Coupling of Aldehydes, Alkynes and Carbamates, Adv. Synth. Catal., 2010, 352, 2437-2440. [link]
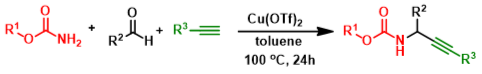
Abstract: A simple and efficient synthesis of propargylcarbamates was developed through a copper(II) triflate-catalyzed coupling of aldehydes, alkynes and carbamates. No co-catalyst or ligand is required.
4. Zhen-Zhen Yang, Liang-Nian He,* Shi-Yong Peng, An-Hua Liu, Lewis Basic Ionic Liquids-Catalyzed Synthesis of 5-Aryl-2-oxazolidinones from Aziridines and CO2 under Solvent-Free Conditions, Green Chem., 2010, 12, 1850-1854. [link]
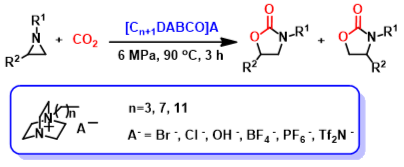
Abstract: A type of DABCO-Derived Lewis basic ionic liquid was developed as a recyclable and efficient catalyst for selective synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2 without utilization of any organic solvent or additive. Therefore, this solvent-free process represents an environmentally friendly process for ionic liquid-catalyzed conversion of CO2 into value-added chemicals.
5. Jian Gao, Liang-Nian He*, Cheng-Xia Miao, Sébastien Chanfreau, Chemical fixation of CO2: efficient synthesis of quinazoline-2, 4-(1H, 3H)-diones catalyzed by guanidines under solvent-free conditions, Tetrahedron, 2010, 66, 4063-4067. [link]

Abstract: Guanidines were proved to be efficient catalysts for the chemical fixation of carbon dioxide with 2-aminobenzonitriles under solvent-free conditions. Notably, the catalysts with low loading worked well for a variety of 2-aminobenzonitriles. As a result, quinazoline-2, 4-(1H, 3H)-diones by employing present protocol were obtained in good yields under mild conditions. This process represents an alternative approach for the greener chemical fixation of CO2 to afford valuable compounds.
6. Zhen-Zhen Yang, Liang-Nian He*, Xiao-Yong Dou, Sébastien Chanfreau, Dimethyl carbonate synthesis catalyzed by DABCO-derived basic ionic liquids via transesterification of ethylene carbonate with methanol, Tetrahedron Lett., 2010, 51, 2931-2934. [link]
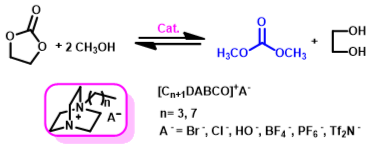
Abstract: Easily prepared, thermal, water and air stable DABCO(1,4-diazobicyclo[2.2.2] octane)-derived basic ionic liquids were proved to be efficient catalysts for DMC synthesis from ethylene carbonate and methanol. [C4DABCO]OH (1-Butyl-4-azo-1-azoniabicyclo[2.2.2]octane hydroxide) showed excellent catalytic activity and 81% DMC yield together with 90% EC conversion was obtained under mild reaction conditions.
7. De-Lin Kong, Liang-Nian He,* Jin-Quan Wang, Synthesis of Urea Derivatives from CO2 and Amines Catalyzed by Polyethylene Glycol-Supported Potassium Hydroxide without Dehydrating Agents, Synlett, 2010, (08), 1276-1280. [link]
Abstract: Polyethylene glycol supported potassium hydroxide (KOH/PEG1000) was developed as a recyclable catalyst for facile synthesis of urea derivatives from amines and CO2 without utilization of additional dehydrating agents. Primary aliphatic amines, secondary aliphatic amines, and diamines can be converted into the corresponding urea derivatives in moderate yields. Furthermore, the catalyst can be recovered after a simple separation procedure, and reused over 5 times with retention of high activity.
8. Xiao-Yong Dou, Liang-Nian He,* Zhen-Zhen Yang, Jing-Lun Wang, Catalyst-Free Process for the Synthesis of 5-Aryl-2-Oxazolidinones via Cyclo-addition Reaction of Aziridines and Carbon Dioxide, Synlett, 2010, (14), 2159-2163. [link]
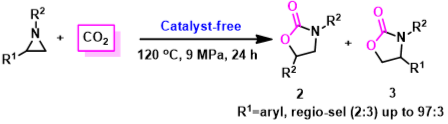
Abstract: A simple approach for facile synthesis of 5-aryl-2-oxazolidinones in excellent regio-selectivity from aziridines under compressed CO2 conditions was developed in the absence of any catalyst and organic solvent. The reaction outcome was found to be tuned by subtly adjusting CO2 pressure. The adduct formed in situ of aziridine and CO2 is assumed to act as a catalyst in this reaction, which was also studied by means of in situ FT-IR technique.
9. De-Lin Kong, Liang-Nian He*, Jin-Quan Wang, Facile synthesis of oxazolidinones catalyzed by n-Bu4NBr3/n-Bu4NBr directly from olefins, chloramine-T and carbon dioxide, Catal. Commun., 2010, 11, 992-995. [link]
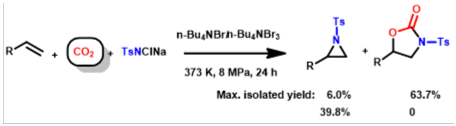
Abstract: A binary catalyst system composed of n-Bu4NBr3/n-Bu4NBr was developed for facile synthesis of 5-substituted 2-oxazolidinones with perfect regioselectivity in a single operation directly from olefins, chloramine-T and CO2.
10. An-Hua Liu, Liang-Nian He*, Shi-Yong Peng, Zhong-Da Pan, Jing-Lun Wang, Jian Gao, Environmentally benign chemical fixation of CO2 catalyzed by the functionalized ion-exchange resins, Sci. China Chem., 2010, 53(7), 1578-1585. (invited by editor) .[link]
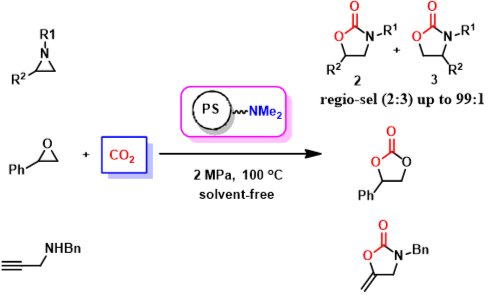
Abstract: Basic ion-exchange resins, one kind of polystyryl supported tertiary amines, were proved to be highly efficient and recyclable catalysts for the fixation of carbon dioxide with aziridines under mild conditions, leading to formation of 5-aryl-2-oxazolidinone with excellent regio-selectivities. Notably, neither solvent nor any additives were required, and the catalyst can be recovered by simply filtration and directly reused at least five times without significant loss of catalytic activity and selectivity. To be delighted, the present protocol was successfully applied to reactions of epoxides /propargyl amines with CO2/CS2. In general, this solvent-free process thus represents environmentally friendly catalytic conversion of CO2 into value-added chemicals and has great potential to be applied in various continuous flow reactors in industry.
11. Fang Wu, Xiao-Yong Dou, Liang-Nian He*, and Cheng-Xia Miao, Natural Amino Acid-Based Ionic Liquids as Efficient Catalysts for the Synthesis of Cyclic Carbonates from CO2 and Epoxides under Solvent-Free Conditions, Lett. Org. Chem., 2010, 7(1), 73-78. [link]
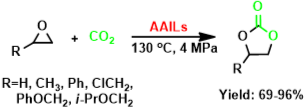
Abstract: Natural α-amino acids-derived ionic liquids comprising 1-butyl-3-methylimidazolium cation and amino acid anion, i.e. [bmim][AA] were found to be effective catalysts for the coupling of various epoxides and CO2 to produce cyclic carbonates in good yields and selectivity, which requires no additional organic solvent and avoids halogen.
12. Liang-Nian He*, Zhen-Zhen Yang, An-Hua Liu, Jian Gao, CO2 Chemistry at Nankai Group: Catalytic Conversion of CO2 into Value-Added Chemicals, ACS Series Book “Advances in CO2 Conversion and Utilization” 2010, Chapter 6, pp77-101(Book chapter invited by editor) [link]
13. 高健,苗成霞,汪靖伦,何良年*,二氧化碳资源化利用的研究进展, 石油化工,2010,39(5), 465-475. (invited by editor) [link]
14. 刘安华,何良年*,高健,杨珍珍,李雨浓,李斌,於兵,二氧化碳化学:二氧化碳的催化转化反应,合成化学 (全国第16届有机和精细化工中间体学术交流会),2010,增刊,80-91.[link]
15. Liang-Nian He, Zhen-Zhen Yang, An-Hua Liu, Jian Gao, Sébastien Chanfreau, Green process for dimethyl carbonate synthesis starting from carbon dioxide, Prep. Pap.-Am. Chem. Soc., Div. Fuel Chem. 2010, 55 (2), 910-911.